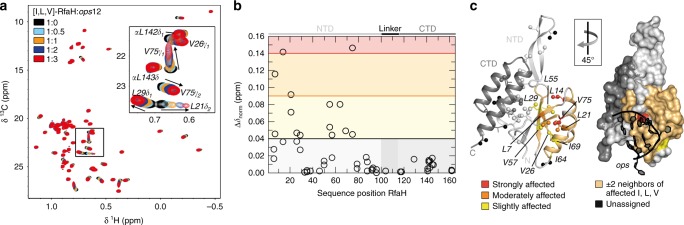
Fig. 2.
RfaH:ops interaction. a 2D [1H, 13C] methyl-TROSY spectra of 45 µM [I,L,V]-RfaH titrated with ops (concentration of stock solution: 1.3 mM). Inset: enlargement of boxed region. b Interaction of [I,L,V]-RfaH with ops. Methyl-TROSY-derived normalized chemical shift changes vs. sequence position of RfaH (corresponding spectra are depicted in a). Horizontal lines: significance levels of Δδnorm = 0.04 ppm, black; = 0.09 ppm, orange, = 0.14 ppm, red. Source data are provided as a Source Data file. c ops binding surface of RfaH as derived from the titration of [I,L,V]-RfaH with ops. Affected methyl groups are mapped onto the RfaH:ops9 structure (PDB ID: ’5OND’). RfaH is shown in ribbon (left) and surface (right) representation (RfaH-NTD, light gray; RfaH-CTD, dark gray), ops9 in ribbon representation (black) with nucleosides as sticks. The arrow indicates how the structures are rotated with respect to each other. Termini are labeled. For graphical representation of the interaction site the whole amino acid is colored. Ile, Leu, and Val residues are shown as sticks with the carbon atoms of the methyl groups as spheres. Slightly affected (0.04 ≤ Δδnorm < 0.09 ppm), yellow; moderately affected (0.09 ≤ Δδnorm < 0.14 ppm), orange; strongly affected (Δδnorm ≥ 0.14 ppm), red; unaffected, colored according to their domain; not assigned methyl groups, black. Two amino acids on either side of an affected Ile/Leu/Val residue are highlighted in beige unless they were unaffected Ile/Leu/Val residues