Figure 1.
Rare Human Variants in SEMA3A-G Disrupt Protein Secretion and Signaling
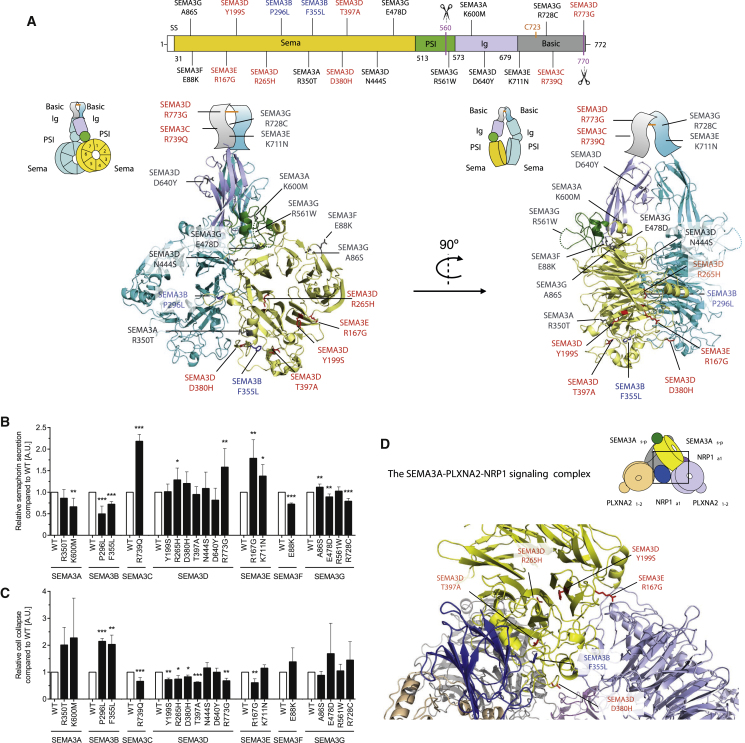
(A) Structural modeling of SEMA3 variants. Upper panel: SEMA3 variants on a schematic representation (mouse Sema3A numbering). SS, signal sequence; Sema, semaphorin domain; PSI, plexin-semaphorin-integrin domain; conserved furin cleavage sites indicated by scissors; conserved cysteines that form SEMA3A-G dimers (orange line). Lower panel: SEMA3A-G mutants mapped onto human SEMA3A structure (increase, blue; decrease, red; no effect, gray; on U87MG cell collapse). Sema and PSI domains on mouse Sema3A crystal structure (PDB: 4GZ8); Ig domain, model combining human SEMA4D (PDB: 1OLZ) and mouse Sema3A (PDB: 4GZ8) structural data; c-terminal basic domain, schematic.
(B) ELISA analysis of C-FLAG-tagged WT/mutant SEMA3A-G secreted in the medium (a.u., arbitrary units).
(C) Effect of WT/mutant SEMA3A-G on cell collapse normalized to amount of semaphorin secreted.
(D) Structural analysis of SEMA3 mutants affecting cell collapse (increased, blue; decreased, red). Mutants are mapped on the crystal structure of the mouse Sema3A-Nrp1-PlxnA2 complex (PDB: 4GZA).
Data represented as mean ± SEM from at least three independent experiments. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 for all experiments.