FIGURE 1.
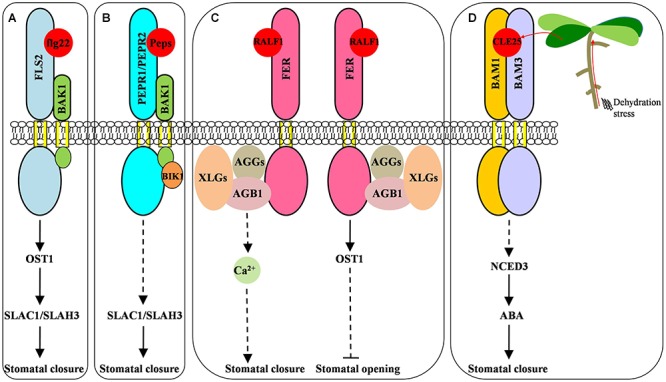
Small signaling peptides-mediated stomatal movement. (A) Pathogen-derived flg22 induced stomatal closure and stimulated the anion channels in an OST1-dependent manner. Upon flg22 perception, the FLS2 receptor interacts with the coreeptor BAK1 to activate OST1 which could phosphorylate the anion channel SLAC1 in guard cells. Additionally, the flg22-induced cytosolic Ca2+ elevation could activate SLAC1 and SLAH3. The activation of anion channels SLAC1 and SLAH3 release anions into the guard cell wall, thereby depolarizing the plasma membrane to induce stomatal closure. (B) The AtPeps-PEPR signaling pathway close stomata by activating guard cell anion channels SLAC1/SLAH3 in an OST1-independent manner. (C) The RALF1-FER signaling module mediated stomatal opening and closure through distinct pathways. All of the G protein subunits, except GAP1, are involved in the induction of stomatal closure and the inhibition of stomatal opening triggered by RALF1. RALF1-FER signaling may trigger cytosolic Ca2+ elevation, which induces stomatal closure. OST1 is only involved in the RALF1 inhibition of stomatal opening, but not in the RALF1 promotion of stomatal closure. (D) The CLE25 peptide-mediated root-to-shoot signaling stimulated the stomatal closure under dehydration stress. Dehydration stress induces CLE25 expression in roots. The resultant synthesized peptide is transported to the leaves, where it binds BAM1/BAM3 and subsequently stimulates ABA accumulation through activating NCED3 expression; this in turn induces stomatal closure. Dashed lines represent missing step(s)/component(s) that are yet-undetermined in the signaling pathway.
