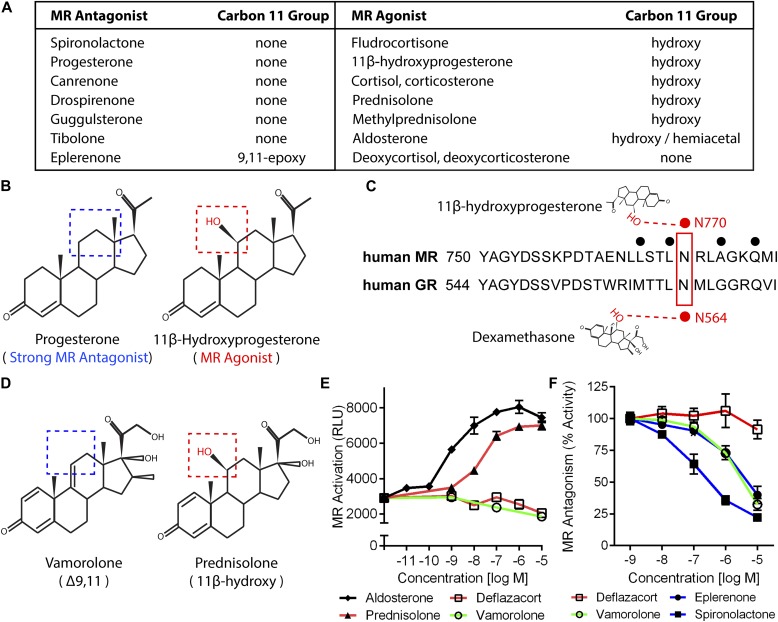
Figure 1. Vamorolone and MR antagonists lack 11-β hydroxyl groups linked to MR activation.
(A) Table of pharmacological and physiological MR ligands with their carbon 11 group identity provided. (B) Progesterone is a potent MR antagonist, whereas addition of an 11β-hydroxy (11β-Hydroxyprogesterone) results in an agonist compound. (C) The 11β-hydroxy group of hydroxyprogesterone interacts with MR residue N770 via hydrogen bonding. Dexamethasone also interacts with this conserved residue in the GR (N564) via hydrogen bonding. (D) Vamorolone is a Δ9,11 steroid where the 11β position features a carbon–carbon double bond, whereas prednisolone is an 11β-hydroxysteroid. (E) A stable MR reporter cell line was treated with drugs and quantified via chemiluminescence assay to determine their agonist properties. Prednisolone and aldosterone showed MR agonist activity. (F) Reporter cells were treated with drug in combination with a constant E80 dose of aldosterone to determine antagonist properties. Vamorolone acted as an MR antagonist, consistent with eplerenone and spironolactone. (Representative data from three experiments with each dose performed in triplicate; values are mean ± SEM).