Figure 1.
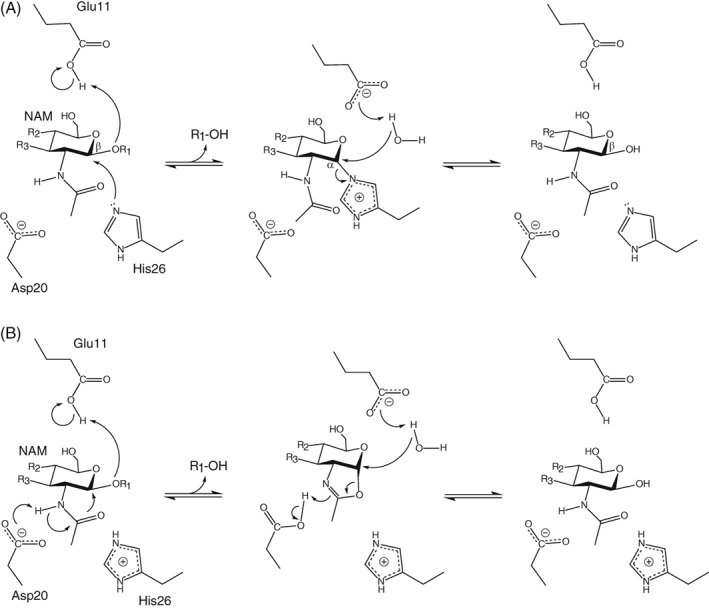
(A) Proposed double‐displacement retaining mechanism for the hydrolysis reaction catalyzed by T26H–T4L.7, 12 (R1 and R2 correspond to NAG; R3 to the peptide‐linked lactyl substituent of NAM.) A minor population of the enzyme is depicted with the hypothesized nucleophile His26 and the general acid/base Glu11 in their catalytically competent protonation states. (B) An alternative mechanism involving substrate‐assisted catalysis. Asp20 is shown as a general base, but could also electrostatically stabilize a charged oxazolinium intermediate without transfer of the N‐acetyl nitrogen‐bonded proton.
