Figure 4.
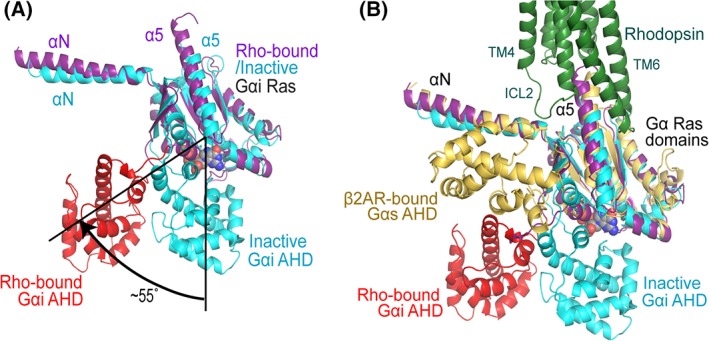
Structural dynamics of the α‐helical domain of G proteins. (A) The α‐helical domain (AHD, red) of the Gαi subunit is 55° tilted away from the Ras domain (magenta) compared with its inactive GDP bound conformation (blue), in which AHD and Ras domain are close to each other to form the nucleotide binding pocket. (B) The comparison of the AHD of Gαi in rhodopsin‐bound conformation with that of Gαs in β2AR‐bound conformation. Gαs is colored in yellow; the same color code as in Panel A is used for rhodopsin‐bound and inactive Gαi subunits.
