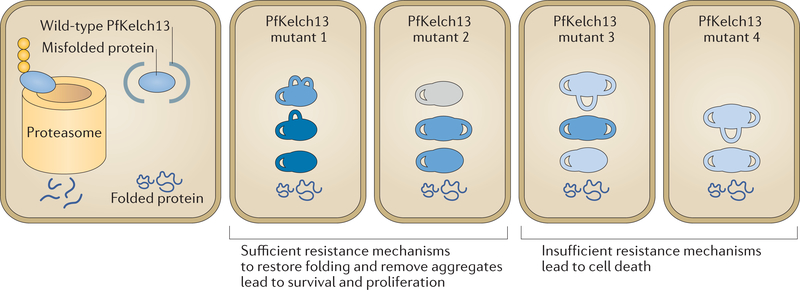
Figure 4 |. A working model for heterogeneity in levels of artemisinin resistance.
In wild-type parasites, in addition to properly folded proteins, misfolded or poorly folded proteins are also made. These ill-folded proteins are bound by Plasmodium falciparum Kelch 13 (PfKelch13), ubiquitylated and targeted to the proteasome for degradation, which keeps their levels low in the parasite. In PfKelch13 mutants, in the absence of ubiquitylation and protein quality control, PfKelch13 substrates (blue) accumulate with varying levels of folding and activity (shown by numbers of loops and intensity of blue colour, respectively). PfKelch13 substrates such as P. falciparum phosphatidylinositol 3-kinase (PfPI3K) may function in pathways that restore protein folding or remove aggregates to mitigate the toxicity induced by the drug. Therefore, genotypically identical parasites have heterogeneous resistance mechanisms to remove the toxic misfolded aggregates induced by artemisinins. As such, following artemisinin-induced toxicity due to protein alkylation and oxidation, only parasites with sufficient resistance mechanisms (which allow clearance of toxic intermediates and restoring of protein folding) survive and proliferate.