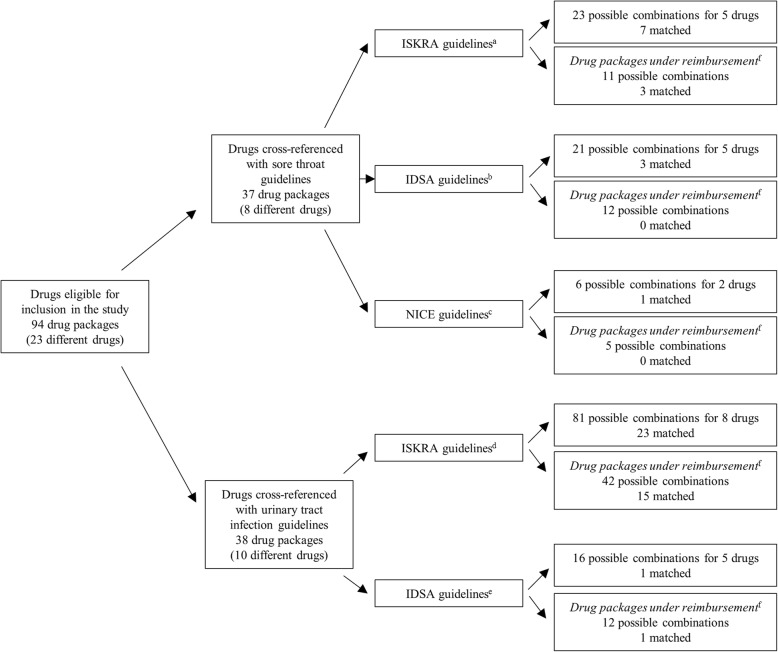
Fig. 2.
Number of drug packages that would in case of accordance with the regimen proposed by the selected guidelines result in 0 excess units (matched). aThe Intersectoral Society for Antibiotic Resistance Control guidelines on sore throat: diagnostic and therapeutic approach and guidelines on antimicrobial treatment and prophylaxis of urinary tract infections. bClinical Practice Guideline for the Diagnosis and Management of Group A Streptococcal Pharyngitis: 2012 Update by the Infectious Diseases Society of America and International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. cNational Institute for Health and Care Excellence Sore throat (acute): antimicrobial prescribing. dThe Intersectoral Society for Antibiotic Resistance Control (ISKRA) guidelines on antimicrobial treatment and prophylaxis of urinary tract infections. eInternational Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. fincluded in Croatian national reimbursement lists on 1st of August 2018