Abstract
Background:
Prenatal overexposure to manganese (Mn), an essential micronutrient, is related to impaired fetal growth and development. Fetuses appear to be highly sensitive to Mn during short periods of gestation. However, little is known about the critical windows of susceptibility to Mn for humans.
Objectives:
Our objective was to estimate trimester-specific associations of exposure to Mn with size at birth.
Methods:
Urine samples of 3,022 women were collected repeatedly in the first, second, and third trimesters in Wuhan, China. Urinary concentrations of Mn and other toxic metals were measured using an inductively coupled plasma mass spectrometry. Trimester-specific associations of specific gravity–adjusted urinary Mn concentrations with birth weight, birth length, and ponderal index were estimated using multivariable linear regressions with generalized estimating equations. Linear mixed models were applied to evaluate the windows of susceptibility to Mn exposure by comparing the pattern of Mn exposure among newborns with restricted size at birth to those without.
Results:
When compared with the third quintile of urinary Mn concentrations, both higher and lower quintiles of urinary Mn concentrations in the second and third trimesters were related to reduced birth weight, birth length, and ponderal index. But the observed associations for higher quintiles were stronger and more likely to be statistically significant [e.g., for women who were in the fifth quintile of Mn concentration in the third trimester, the reduction in birth weight was (95% CI: , ) g and in birth length was (95% CI: , 0.00) cm]. Moreover, newborns with restricted size at birth, compared with those without, had higher levels of Mn exposure in the second and third trimesters.
Conclusions:
This prospective prenatal cohort study revealed an association of exposure to Mn during pregnancy, especially late pregnancy, with restricted size at birth. Replications are needed. https://doi.org/10.1289/EHP3423
Introduction
Manganese (Mn), an abundant element on Earth, is an essential micronutrient for animals and humans and functions as a cofactor in several important biological processes during development, including bone formation, protein and energy metabolism, and metabolic regulation (ATSDR 2012; Erikson et al. 2007; Wood 2009). However, Mn is also a potential toxicant for humans at excessive exposure levels (Crossgrove and Zheng 2004). Environmental Mn occurs naturally and is also released into the atmosphere, waterways, and soil from industrial activities such as mining and metal smelting (ATSDR 2012; He et al. 2005). Mn is widely used in manufacturing a variety of products, including cosmetics, fertilizer, paints, fireworks, and additive agents in steel production and gasoline (ATSDR 2012). The general population is exposed to Mn mainly through diet, but additional amounts of Mn, usually from the environment, can also enter the human body via the respiratory system and skin contact (ATSDR 2012).
The fetal period is particularly sensitive to environmental threats (Barker 2007). Exposure to environmental toxicants during the fetal period have been related to not only restricted fetal growth and development (Cheng et al. 2017; Hu et al. 2017, 2018; Kippler et al. 2012; Kuzawa 2005; Liu et al. 2018; Peng et al. 2018; Xia et al. 2016; B Zhang et al. 2015) but also long-term health challenges (Cupul-Uicab et al. 2013; Gluckman et al. 2008; Vafeiadi et al. 2015). Moreover, identifying critical windows of susceptibility to environmental pollutants exposure during pregnancy is essential for infants’ and children’s health because exposures during such time periods may be associated with stronger health effects (Sánchez et al. 2011). Mn crosses the placental barrier through active transport mechanisms (Krachler et al. 1999). Animal studies have linked both gestational Mn deficiency and overexposure to decreased fetal size (Bolze et al. 1985; Colomina et al. 1996; Sánchez et al. 1993; Treinen et al. 1995). Moreover, mice and rats are sensitive to Mn-related fetotoxicities, including reduced fetal body weight and increased risk of skeleton malformation, during mid (days 9–10) and late gestation (days 15–17) (Colomina et al. 1996; Treinen et al. 1995).
In humans, Mn deficiency is rare because the required amount of Mn can be provided through dietary intake (EFSA NDA Panel 2013). However, excessive exposure to Mn during pregnancy has been related to impaired neurological development (Bouchard et al. 2011; Menezes-Filho et al. 2011). A recent study of the general Chinese population suggested that the Mn exposure level in women was nearly 30% higher than in men (LL Zhang et al. 2015). In addition, one of our previous studies suggested that exposure to higher levels of Mn, even at levels that did not exceed the upper limit of urinary reference () (McClatchey 2002), was associated with an increased risk of low birth weight (Xia et al. 2016). Moreover, a few studies suggested parabolic dose–response relationships between Mn exposure during pregnancy and size at birth, but most of these findings were based on one-time exposure assessments (Chen et al. 2014; Eum et al. 2014; Guan et al. 2014; Mora et al. 2015; Tsai et al. 2015; Xia et al. 2016; Zota et al. 2009). Only two studies, to our knowledge, have investigated trimester-specific associations of Mn exposure during pregnancy with birth outcomes in humans and had limited sample sizes (Mora et al. 2015; Tsai et al. 2015).
Based on a Chinese prospective prenatal cohort of 3,022 women, this study aimed to assess trimester-specific associations of exposure to Mn during pregnancy with size at birth as well as to identify the critical window of heightened susceptibility of Mn exposure.
Methods
Study Population
This study was carried out based on our ongoing prospective prenatal cohort study that was launched in October 2013 at the Wuhan Women and Children Medical Care Center, the municipal health center for women and children in Wuhan, Hubei Province, China. A pregnant woman was eligible if she a) agreed to provide signed informed consent; b) was less than 16-weeks pregnant with a single gestation at the time of enrollment; c) was a resident of Wuhan and knew Chinese; d) agreed to complete in-person interviews, undergo ultrasound examinations, and provide blood and urine samples at government-recommended prenatal care visits; and e) was willing to give birth at the study hospital. Between October 2013 and October 2016, 3,075 women who provided at least one urine sample, did not smoke tobacco during pregnancy, and gave birth to live singletons without birth defects were included. After additionally excluding 53 women with missing data of confounding variables, 3,022 women were retained in this study. The study protocol was reviewed and approved by the ethics committees of Tongji Medical College, Huazhong University of Science and Technology [No. (2012)07] and the Wuhan Women and Children Medical Care Center [No. 2010009].
Urine Collection and Analysis
Maternal urine samples [ (SD)] were obtained at three government-recommended prenatal care visits in the first ( weeks, the first prenatal care visit), second ( weeks), and third ( weeks) trimesters. Of all the study population, 823 (27.2%) women provided one urine sample, 1,342 (44.4%) women provided two urine samples, and 857 (28.4%) women provided three urine samples. It is noteworthy that 2,252 (81.3%) of all the first-trimester urine samples () were obtained at or before 13 weeks of gestation. Women with missing urine samples either rejected providing one, missed their prenatal care visits, or went into early labor before the third-trimester urine sample was collected. Prior to assessments, all urine samples were stored in polypropylene cups at for a median of 46.1 (interquartile range: 36.1–57.4) weeks for first-trimester samples, 35.6 (25.4–46.4) weeks for second-trimester samples, and 26.4 (16.6–36.6) weeks for third-trimester samples. The temperature of all refrigerators was monitored weekly to ensure the condition of sample storage.
We used an inductively coupled plasma mass spectrometry (ICP-MS; Agilent 7,700, Agilent Technologies) to measure urinary concentrations of Mn and other toxic metals that have been related to restricted fetal growth or size at birth in previous studies, including vanadium (V), arsenic (As), cadmium (Cd), and lead (Pb) (Cheng et al. 2017; Hu et al. 2017, 2018; Kippler et al. 2012; Liu et al. 2018; B Zhang et al. 2015). Detailed descriptions for sample preparation, assessment, ICP-MS operating condition, and quality controls have been provided previously (Cheng et al. 2017). Briefly, urine samples were thawed at room temperature, nitrated overnight using 3% , and sonicated by ultrasound at 40°C for 1 h. Before being assessed by the ICP-MS, supernatants were obtained after centrifuging the resulting samples; then , , , , and were monitored in helium mode. The detection limit of quantification (LOQ) of each metal was calculated as . The LOD (limit of detection) was calculated as , where represents the relative standard deviation for 11 measurements of the background intensity, C stands for the concentration of certain metals in the solution, and SBR is the signal-to-background ratio (Boumans et al. 1991). The LOQs were for Mn, for V, for As, for Cd, and for Pb. The detection rates of all these metals were higher than 97%. All available urine samples from each woman were prepared and assessed in one batch. Quality control samples were embedded in each batch and were measured in duplicate. In addition, we used the Standard Reference Material Human Urine (SRM2670a; National Institute of Standards and Technology) as an external quality control, and the spike–recoveries of Mn and other toxic metals ranged from 98.8% to 106.4%. We also evaluated measurement variations of all these metals by measuring quality control samples repeatedly. The intraday variations were between 0.29% and 0.89%, and the interday variations were ranged from 0.27% to 0.98% (see Table S1).
Urinary specific gravity (SG) was analyzed by a refractometer (Atago PAL-3; Atago) at room temperature. Before each assessment, the refractometer was calibrated with deionized water.
Size at Birth
At the time of delivery, birth weight (in grams) and birth length (in centimeters) were measured and recorded by trained nurses in the study hospital under clinical criteria. Ponderal index (in kilograms per cubic meter) was calculated as and was considered an indicator of asymmetrical intrauterine growth retardation (Landmann et al. 2006). Gestational age at birth was calculated as days between the date of delivery and the first day of the last menstrual period (LMP). The dates of LMP were reported by pregnant women and were then corrected by obstetricians using the first-trimester ultrasound measures under clinical criteria. For women who provided accurate dates of the LMP, corrected gestational ages were used if the difference between the reported and corrected gestational ages was . A term birth was defined as a childbirth occurring after 37 weeks of gestation.
We constructed gestational age–adjusted standard deviation scores (SD-scores) for birth size parameters using the GAMLSS package (version 4.3-7) in R (version 3.3.2; R Development Core Team). These SD-scores represent the percentiles of weight, length, or ponderal index at birth. The calculation was based on an assumption that the distributions of these parameters depend only on gestational age. We used Box-Cox transformations to normalize these parameters (Rigby and Stasinopoulos 2005). Then, we modeled each parameter using a cubic spline of gestational age in days. Finally, gestational age–adjusted SD-scores for birth weight, birth length, and ponderal index were generated based on the best fitting models, which were selected according to the Akaike’s information criteria.
Covariates
Trained nurses used standardized and structured questionnaires during in-person interviews at the first prenatal care visits to collect the information on maternal age, education, tobacco smoking, passive smoking, alcohol consumption, folic acid supplementation, physical activity during pregnancy, and paternal height and weight. Maternal prepregnancy and paternal body mass index (BMI; kilograms per cubic meter) were subsequently calculated. Gynecological and obstetrical information (e.g., parity, anemia, hypertensive disorders of pregnancy, gestational diabetes mellitus, and infant sex) was retrieved from medical records. In order to ensure accuracy and reliability, we carried out scheduled and standardized quality controls for the data used in this study.
Statistical Analysis
Urinary metal concentrations (micrograms per liter) below the LOQ for each metal were imputed with LOQs divided by the square root of 2. Urinary concentrations of Mn and other toxic metals were adjusted by urinary SG using: , where represents the SG-adjusted concentration, P represents the unadjusted concentration, 1.011 is the median SG of all measured urine samples, and SG represents the individual urinary specific gravity (Duty et al. 2005). Both SG-adjusted and -unadjusted concentrations were transformed by natural logarithm (ln) to reduce the influence of outliers.
Pearson correlation coefficients for urinary concentrations of Mn and other toxic metals in each trimester were calculated based on ln-transformed concentrations. In order to estimate their temporal variabilities, we used mixed-effects models to calculate intraclass correlation coefficients (ICCs) and 95% confident intervals (CIs) of urinary SG and urinary Mn concentrations. The ICC, ranging from 0 (no reproducibility) to 1 (perfect reproducibility), is the ratio of between-subject variance to total variance and which evaluates the reproducibility of a biomarker in a population over time (Rosner 2000).
We applied a multiple informant model to test trimester-specific associations between urinary Mn concentrations during pregnancy and size at birth (Sánchez et al. 2011). The multiple informant model treats Mn concentrations at different time windows as informants, using linear regressions with generalized estimating equations to estimate associations of each individual Mn concentration with a given outcome simultaneously in the same statistical model. In addition, this multiple informant model does not adjust for exposures in other time windows but instead tests the null hypothesis that the associations of Mn concentrations are equal at each time window versus the alternative hypothesis that at least one association differs from the rest. We used the multiple informant model to estimate the trimester-specific associations of quintiles of urinary Mn concentrations (as categorical variables, the mid-quintiles were set as the references) in the first, second, and third trimesters with birth weight, birth length, and ponderal index. Trimester-specific quintiles were generated based on urinary Mn concentrations in each trimester.
We also used the method described by Sánchez et al. (2011) for additional analysis. This method, based on linear mixed models, compares the prenatal exposure pattern of newborns with restricted size at birth to those without in order to evaluate which period of pregnancy might be the critical window of heightened susceptibility to Mn exposure. Specifically, we used this method to compare the pattern of urinary ln-Mn during pregnancy for newborns with low SD-scores (in the 10th percentiles) for birth weight, birth length, or ponderal index to those with high SD-scores (in the 90th percentiles).
All the statistical models were adjusted for potential confounders, including gestational age at the time of delivery (continuous), maternal age at recruitment (continuous), parity (nulliparous/multiparous), maternal prepregnancy BMI (categorized based on the Chinese standard: ), alcohol consumption before pregnancy (no/yes), active smoking before pregnancy (no/yes), passive smoking during pregnancy (no/yes), education (), folic acid supplementation (no/only in the first trimester/only in the second and third trimester/during the entire pregnancy), physical activity during pregnancy (no/1–4/5–7 d/week), and infant sex (boy/girl). Models for birth length were also adjusted for maternal and paternal height (continuous). In order to test the potential impact of pregnancy complications, models were additionally adjusted for anemia (no/yes), gestational diabetes mellitus (no/yes), and hypertensive disorders of pregnancy (no/hypertension/preeclampsia).
Stratified analyses were conducted to test potential differences between boys and girls and between younger ( old at recruitment) and older ( old at recruitment) women. Stratum-specific p-values for interaction were estimated as the p-values for the interaction terms of infant sex (or maternal age) and each quintile of urinary Mn concentrations. As a sensitivity analysis to examine the impact of premature deliveries, we replicated all the models restricted to term births. Another sensitivity analysis was conducted to determine whether there was residual confounding from correlated metal exposures and whether the observed associations in this study were false positives by replicating regression models with additional adjustment for other toxic metals (V, As, Cd, and Pb) that have been related to restricted fetal growth or size at birth (Cheng et al. 2017; Hu et al. 2017, 2018; Kippler et al. 2012; Liu et al. 2018; B Zhang et al. 2015). In the third sensitivity analysis, we replicated regression models restricted to women without anemia, gestational diabetes mellitus, or hypertensive disorders of pregnancy in order to evaluate the impact of maternal pregnancy complications. Finally, we replicated regression models a) restricted to women who provided three urine samples to evaluate the potential misclassification caused by missing of urine samples, b) using unadjusted concentrations in regression models to assess the potential misclassification caused by urine dilution, and c) using the quintile of Mn concentration that was generated based on all measurements to estimate the potential misclassification caused by variations of urinary Mn concentrations throughout the whole pregnancy.
All statistical analyses were performed using R (version 3.3.2) or SAS (version 9.4; SAS Institute, Inc.). The statistical significance level was 0.05 for a two-tailed test.
Results
Of all the 3,022 women in this study, 2,923 gave birth at term. Women recruited in this study were on average of y of age, and most of them were nulliparous (85.4%), nonsmokers (99.4%), and nondrinkers (99.2%). Before pregnancy, 575 (19.0%) women were underweight (), 323 (10.7%) were overweight (BMI between 24.0 and ), and only 65 (2.2%) were obese (). Most of the women had at least a college-level education (78.6%), used folic acid supplementations during pregnancy (83.5%), or exercised at least 5 d a week during pregnancy (75.1%). In addition, approximately 52.7% of the newborns were boys (Table 1). The distributions of maternal, paternal, and neonatal baseline characteristics for term births were similar to those for the whole population (see Table S2).
Table 1.
Characteristics of parents and children in the study population (Wuhan, China).
| Characteristic | (%) or |
|---|---|
| All individuals | 3,022 |
| Maternal characteristics | |
| Age at recruitment (y) | |
| 398 (13.17) | |
| 25–30 | 1,833 (60.66) |
| 30–35 | 655 (21.67) |
| 136 (4.50) | |
| Prepregnancy BMI () | |
| 575 (19.03) | |
| 18.5–23.9 | 2,059 (68.13) |
| 24.0–27.9 | 323 (10.69) |
| 65 (2.15) | |
| Height (m) | |
| Parity | |
| Nulliparous | 2,580 (85.37) |
| Multiparous | 442 (14.63) |
| Anemia during pregnancy | |
| No | 3,003 (99.37) |
| Yes | 19 (0.63) |
| Hypertensive disorders of pregnancy | |
| No | 2,926 (96.82) |
| Hypertension | 63 (2.08) |
| Preeclampsia | 33 (1.09) |
| Gestational diabetes mellitus | |
| No | 2,760 (91.33) |
| Yes | 262 (8.67) |
| Active smoking before pregnancy | |
| No | 3,003 (99.37) |
| Yes | 19 (0.63) |
| Passive smoking during pregnancy | |
| No | 2,142 (70.88) |
| Yes | 880 (29.12) |
| Alcohol consumption before pregnancy | |
| No | 2,997 (99.17) |
| Yes | 25 (0.83) |
| Education | |
| Compulsory and lower () | 194 (6.42) |
| High school and equivalent (9–12 y) | 453 (14.99) |
| Graduate and higher () | 2,375 (78.59) |
| Folic acid supplementation | |
| No | 499 (16.51) |
| Only in the 1st trimester | 1,047 (34.65) |
| Only in the 2nd and 3rd trimester | 620 (20.52) |
| During the entire pregnancy | 856 (28.33) |
| Physical activity during pregnancy | |
| No | 278 (9.20) |
| 1–4 d/week | 476 (15.75) |
| 5–7 d/week | 2,268 (75.05) |
| Paternal height (m) | |
| Neonatal characteristics | |
| Infant sex | |
| Male infant | 1,594 (52.75) |
| Female infant | 1,428 (47.25) |
| Gestational age (weeks) | |
| Birth weight (g) | |
| Birth length (cm) | |
| Ponderal index () |
Note: BMI, body mass index; SD, standard deviation.
The geometric mean of SG-adjusted urinary Mn concentrations in all urine samples was (95% CI: 1.42, 1.50) and the median was (95% CI: 1.37, 1.45; see Table S1). Urinary Mn concentrations of the study population were compared with those of other populations (Table 2). Pearson correlation coefficients of urinary Mn concentrations and other toxic metals were between 0.07 and 0.57 (see Table S3). The ICC (95% CI) of SG-adjusted urinary ln-Mn was 0.32 (95% CI: 0.29, 0.35) in all urine samples, indicating a poor reproducibility throughout the whole pregnancy. The ICC in the first and third trimesters (, 95% CI: 0.25, 0.34) was lower than that in the first and second trimesters (, 95% CI: 0.29, 0.38), as well as that in the second and third trimesters (, 95% CI: 0.29, 0.40). In addition, the reproducibility of unadjusted urinary ln-Mn in all urine samples [ (95% CI: 0.36, 0.42)] was slightly higher than SG-adjusted concentrations. Compared with urinary ln-Mn, urinary SG [ (95% CI: 0.13, 0.20)] had a poorer reproducibility throughout the whole pregnancy.
Table 2.
The distributions of urinary Mn concentrations () during pregnancy.
| Population and location | Urine samples | Sample size (n) | Urine Mn concentration () | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AM | GM | Percentiles | |||||||
| 5th | 25th | 50th | 75th | 95th | |||||
| Present study: Pregnant women in an urban city in Hubei, China, 2013–2016; mean 27.8 y of age (18–44 y) | Spot, 13.0 weeks | 2,771 | 2.55 | 1.44 | 0.33 | 0.80 | 1.45 | 2.57 | 6.19 |
| Spot, 23.3 weeks | 1,481 | 2.01 | 1.21 | 0.27 | 0.72 | 1.16 | 2.03 | 5.18 | |
| Spot, 37.8 weeks | 1,826 | 2.50 | 1.47 | 0.31 | 0.82 | 1.47 | 2.69 | 7.17 | |
| Spot, 13.0 weeks (SG adjusted) | 2,771 | 2.89 | 1.38 | 0.26 | 0.68 | 1.35 | 2.65 | 9.89 | |
| Spot, 23.3 weeks (SG adjusted) | 1,481 | 2.72 | 1.38 | 0.28 | 0.70 | 1.29 | 2.57 | 8.24 | |
| Spot, 37.8 weeks (SG adjusted) | 1,826 | 3.69 | 1.66 | 0.28 | 0.83 | 1.63 | 3.08 | 11.5 | |
| Xia et al. (2016): Pregnant women in urban and rural cities in Hubei, China, 2012–2014; mean 28.1 y of age (17–42 y) | Spot, hospital admission for delivery | 816 | 0.66 | — | 0.13 | 0.38 | 0.85 | — | |
| Callan et al. (2013): Pregnant women in western Australia, 2008–2011; mean 32.0 y of age (19–44 y) | First morning void, 2 weeks prior to delivery | 173 | 1.00 | — | 0.10 | — | 0.33 | — | 3.23 |
| Gunier et al. (2014): Pregnant women in an agricultural community in California, 1999–2000; (70% of age) | Spot, 26 weeks | 59 | — | 0.50 | — | 0.20 | 0.40 | 0.60 | — |
| Ljung et al. (2009): Pregnant women in a poor rural subdistrict in Bangladeshi, 2002; mean 27.0 y of age (14–44 y) | Spot, 14 weeks | 388 | 2.50 | — | — | 0.90 | 1.60 | 2.80 | — |
| CDC (2017): U.S. residents (NHANES study), 2011–2012 | Spot, females | 1,242 | — | 0.13 | — | — | 0.12 | 0.21 | 0.41 |
| Spot, nonsmokers 20–49 y of age | 671 | — | 0.12 | — | — | 0.11 | 0.19 | 0.36 | |
| Spot, nonsmoking women | 708 | — | 0.12 | — | — | 0.12 | 0.20 | 0.36 | |
| Health Canada (2010): Canadian women (CHMS), 2007–2009 | Spot, 6–79 y of age | 2,792 | 0.15 | 0.08 | 0.09 | 0.17 | 0.41 | ||
| Spot, 20–39y of age | 643 | 0.13 | 0.08 | 0.08 | 0.17 | 0.40 | |||
| Spot, 40–59y of age | 643 | 0.15 | 0.08 | 0.09 | 0.16 | 0.40 | |||
| Ohashi et al (2006): Women in Japan, 2000–2005; mean 47.5 y of age (20–81 y) | Spot | 1,000 | — | 0.14 | — | — | 0.16 | — | — |
| Spot (SG adjusted) | 1,000 | — | 0.12 | — | — | 0.12 | — | — | |
| Goullé et al. (2005): Men and women in France (age and year not specified) | Spot | 100 | — | — | 0.11 | — | 0.31 | — | 1.32 |
| Heitland and Köster (2006): Men and women in Germany, 2005; 18–65 y of age | Morning middle-stream | 87 | 0.09 | 0.06 | — | — | — | — | 0.21 |
Note: AM, arithmetic mean; CHMS, Canadian Health Measures Survey; GM, Geometric mean; NHANES, National Health and Nutrition Examination Survey; SG, specific gravity.
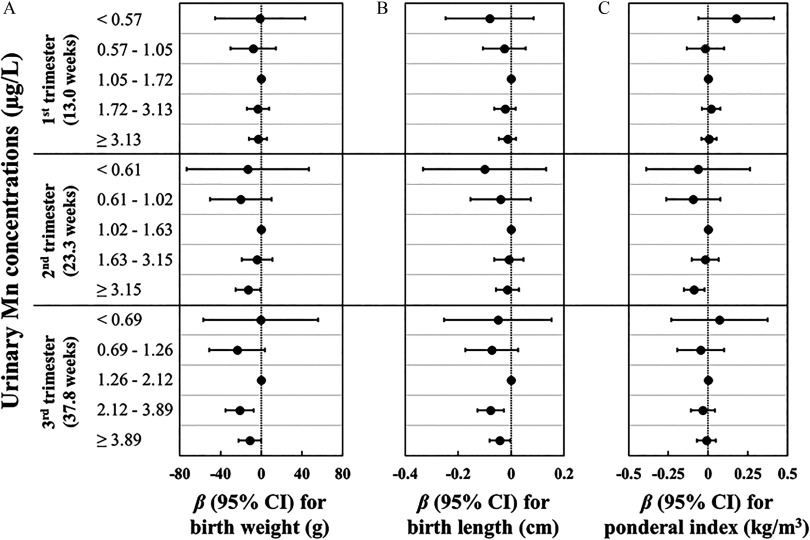
In multivariable analyses, we did not observe any association of urinary Mn concentration in the first trimester with size at birth. When comparing higher quintiles to the third quintile of urinary Mn concentrations in the second and third trimesters, we observed significant reductions in birth weight, birth length, and ponderal index [e.g., for women who were in the fourth and fifth quintiles of Mn concentrations in the third trimester, the reduction in birth weight was (95% CI: , ) g and (95% CI: , ) g, respectively, and the reduction in birth length was (95% CI: , ) cm and (95% CI: , 0.00) cm, respectively]; whereas nonsignificant reductions in birth weight, birth length, and ponderal index were observed when comparing lower quintiles of Mn concentrations (Figure 1; see also Table S4). Of note, when restricting the analysis to term births, the associations of urinary Mn concentrations in different trimesters with size at birth were similar with those observed in all participants (see Table S5). Further adjusting for pregnancy complications or other toxic metals (see Table S4), or limiting the analysis to women without pregnancy complications (see Table S6) yielded generally similar findings. In addition, compared with the main results, the observed associations were slightly attenuated when restricting the analysis to women who provided three urine samples (see Table S7), categorizing quintiles of urinary Mn concentration by pooling measurements from all trimesters (see Table S8), or using unadjusted urinary Mn concentrations (see Table S9). However, association directions remained consistent with the main results.
Figure 1.
Adjusted associations of specific gravity (SG)–adjusted urinary Mn concentrations () during pregnancy with size at birth. Regression coefficients () and 95% confidence intervals (CIs) for associations of SG-adjusted urinary Mn concentrations during pregnancy with (A) birth weight, (B) birth length, and (C) ponderal index were estimated using linear regressions with generalized estimating equations. Models were adjusted for gestational age at delivery, maternal age at recruitment, parity, prepregnancy body mass index, alcohol consumption before pregnancy, active smoking before pregnancy, passive smoking during pregnancy, education, folic acid supplementation, physical activity during pregnancy, and infant sex. The model for birth length model was additionally adjusted for maternal and paternal height. Numeric data are available in Table S4.
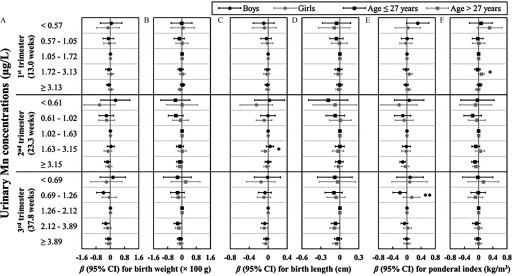
In stratified analyses (Figure 2; see also Table S10), infant sex-based differences in associations between urinary Mn concentrations and size at birth were generally nonsignificant (). Most of the associations observed in boys were similar to those observed in girls; however, some were in the opposite directions [e.g., when comparing the second quintile to the third quintile of urinary Mn concentrations in the third trimester, the ponderal index for boys reduced by (95% CI: , ) , but the ponderal index for girls increased by 0.13 (95% CI: , 0.37) , ]. Otherwise, when stratified by maternal age at recruitment, we did not observe age-based differences in associations between urinary Mn concentrations and size at birth.
Figure 2.
Adjusted associations of specific gravity (SG)–adjusted urinary Mn concentrations () during pregnancy with size at birth, stratified by infant sex and maternal age at recruitment. Regression coefficients () and 95% confidence intervals (CIs) for associations of SG-adjusted urinary Mn concentrations during pregnancy with (A,B) birth weight, (C,D) birth length, and (E,F) ponderal index were estimated by applying linear regressions with generalized estimating equations and were stratified by infant sex (A,C,E) or maternal age at recruitment (B,D,F). Models were adjusted for gestational age at delivery, maternal age at recruitment, parity, prepregnancy body mass index, alcohol consumption before pregnancy, active smoking before pregnancy, passive smoking during pregnancy, education, folic acid supplementation, physical activity during pregnancy, and infant sex. Models for birth length model were additionally adjusted for maternal and paternal height. Stratum-specific p-values for interaction were estimated as the p-values for the interaction terms of infant sex (or maternal age) and each quintile of urinary Mn concentrations. Numeric data, including stratum-specific p-values for interaction, are available in Table S10. *; **.
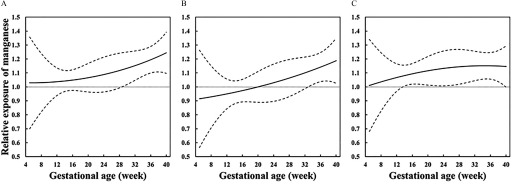
In the analyses of critical windows of susceptibility to Mn (Figure 3), we observed that relative exposure levels of Mn for newborns who were in the 10th percentiles of birth weight, birth length, or ponderal index increased throughout the whole pregnancy, compared with those who were in the 90th percentiles, suggesting that the inverse association of urinary Mn concentrations with size at birth became stronger as the pregnancy progressed. In addition, for newborns who were born with a birth weight in the 10th percentile, compared with those in the 90th percentile, the relative exposure levels of Mn became significantly higher from 29 weeks of gestation. As of for birth length and ponderal index, relative exposure levels of Mn were significantly higher after 33 weeks and 14 weeks of gestation, respectively. Furthermore, when restricting the analysis to term births (see Figure S1) or women who provided three urine samples (see Figure S2), the observed exposure patterns of Mn were consistent with those observed in the whole population.
Figure 3.
Relative exposure levels of Mn comparing newborns with restricted size at birth to those without. Solid lines represent relative exposures of Mn through the whole pregnancy, comparing newborns in the 10th percentiles of (A) birth weight, (B) birth length, or (C) ponderal index to those in the 90th percentiles. Dotted lines represent pointwise 95% confidence intervals. Exposure patterns of Mn during pregnancy were estimated using linear mixed models with adjustment for gestational age at delivery, maternal age at recruitment, parity, prepregnancy body mass index, alcohol consumption before pregnancy, active smoking before pregnancy, passive smoking during pregnancy, education, folic acid supplementation, physical activity during pregnancy, and infant sex. The model for birth length model was additionally adjusted for maternal and paternal height.
Discussion
This prenatal cohort study investigated the trimester-specific associations of Mn exposure with size at birth in a large population of 3,022 women who were recruited in China. Based on repeated measurements of urinary Mn concentrations in the first, second, and third trimesters, we observed significant associations of elevated urinary Mn concentrations during pregnancy, especially in the second and third trimesters, with reductions in birth weight, birth length, and ponderal index. Moreover, newborns with restricted size at birth had relatively higher exposure levels of Mn in the second and third trimesters, especially during the late third trimesters.
In the study population, based on SG-adjusted urinary Mn concentrations, only 309 (5.1%) urine samples that were collected in different time periods exceeded the upper limit of the urinary reference for adults () (McClatchey 2002). Urinary Mn concentrations of women in this study were lower than, but comparable to, those of pregnant women in a poor rural subdistrict in Bangladesh (Ljung et al. 2009). However, urinary Mn concentrations of the study population were higher than that of the subpopulation in our previous study (Xia et al. 2016), of pregnant women from an agricultural community in California (Gunier et al. 2014), and of nonsmoking pregnant women from Western Australia (Callan et al. 2013). Similarly, urinary Mn concentrations in the study population were relatively higher than those of adult men and nonpregnant women from different countries, including the United States (CDC 2017), Canada (Health Canada 2010), Japan (Ohashi et al. 2006), France (Goullé et al. 2005), and Germany (Heitland and Köster 2006). The differences in urinary Mn concentrations across populations could be a result of different environmental exposure levels and dietary intakes. In addition, the Chinese population consumes more plant foods, which are main sources of dietary Mn, than the U.S. and European populations (Zhou et al. 2016). Therefore, our findings should be interpreted and generalized with caution.
In this study, we observed that the ICC of urinary Mn concentrations indicated a poor reproducibility throughout the whole pregnancy. In addition, the median urinary Mn concentration in the third trimester was higher than that in the first and second trimesters, which was consistent with previous studies, suggesting Mn concentrations in maternal blood increases with the progression of gestation (Mora et al. 2015; Takser et al. 2004; Tholin et al. 1993; Tsai et al. 2015). Because fetal development may require additional amounts of Mn and lead to increased intestinal absorption or tissue Mn mobilization (Kirchgessner et al. 1982), which may result in late-pregnancy increases in urinary Mn concentrations, variations of Mn exposure levels during pregnancy, together with these physiological changes, could both contribute to the low reproducibility of urinary Mn concentrations throughout the whole pregnancy. Therefore, one time-point exposure estimation does not accurately represent Mn exposure levels in the whole gestation, and it is necessary to identify the window of heightened susceptibility to Mn for fetal growth. Because of the poor reproducibility of urinary Mn concentrations, it would cause potential misclassifications when using overall quintiles of urinary Mn concentrations to estimate the relationship between urinary Mn concentrations and size at birth. Therefore, we generalized trimester-specific quintiles for urinary Mn concentrations in each trimester. Furthermore, in order to reduce the influence of potential information bias caused by urine dilution, we used urinary SG instead of creatinine to adjust for urinary concentrations of Mn and other metals because urinary creatinine concentration increases as the pregnancy progresses due to physiological and anatomical changes in renal function for pregnant women (Cheung and Lafayette 2013).
We set medium quintiles of urinary Mn concentrations as reference groups in different time periods because the reference values of urinary or blood Mn concentrations during pregnancy have not been defined. Recent studies reported potential inverted U-shaped relationships between maternal blood Mn concentrations and birth weight (Chen et al. 2014; Eum et al. 2014; Zota et al. 2009). Another study from Dalian, China, observed a potential parabolic dose–response relationship between Mn concentrations in umbilical cord blood and birth weight (Guan et al. 2014). In addition to the above studies, our previous study also observed a potential U-shaped relationship between urinary Mn concentration and low birth weight risk (Xia et al. 2016). In this study, we also observed potential inverted U-shaped associations of urinary Mn concentrations in early, mid-, and late pregnancy with birth weight, birth length, and ponderal index: Higher maternal urinary Mn concentrations were related to stronger and more significant associations with reduced size at birth than lower concentrations.
Identifying critical windows of susceptibility to environmental pollutants exposure during pregnancy is essential for infants’ and children’s health because environmental exposures in such time windows could bring stronger impairment in children’s growth and health (Sánchez et al. 2011; Selevan et al. 2000). An animal study suggested that prenatal exposure to Mn was related to reduced fetal body weight and increased incidences of skeletal defects in mice, and exposures on days 9 and 10 of gestation (midpregnancy) had the strongest fetotoxicity (Colomina et al. 1996). Another experimental study indicated that exposure to Mn from days 15 to 17 of gestation (late pregnancy) had the highest incidence of dose-dependent skeletal malformations in rats (Treinen et al. 1995). To our knowledge, only two studies have investigated the trimester-specific associations of exposure to Mn with size at birth in humans, but the findings were inconsistent. One study, from Costa Rica (), observed linear associations of maternal hair Mn concentrations in the second and third trimesters with reduced chest circumference at birth (Mora et al. 2015). The other study, based on 76 women from Taiwan, suggested that Mn concentration in maternal erythrocytes in the first and second trimesters were linearly associated with reduced birth weight and neonatal head and chest circumferences (Tsai et al. 2015). However, both studies were conducted with limited sample sizes and did not investigate the nonlinear associations. In this study, based on a large sample size of 3,022 women, we observed that urinary Mn concentrations in the second and third trimesters had stronger associations with reduced size at birth, which were consistent with those from animal studies and the study from Costa Rica (Mora et al. 2015). These findings suggest that mid and late trimesters may be critical windows of susceptibility to Mn exposure for fetal growth.
Dietary intake is the main source of Mn exposure for humans (ATSDR 2012). A plant-based dietary pattern might be related to Mn exposure levels because rice, grains, soy beans, nuts, vegetables, fruits, and tea are rich in Mn (Zhou et al. 2016). In animal studies, maternal exposure to high levels of Mn through oral administration resulted in reduced fetal weight, impaired skeleton ossification, and restricted internal organ development in offspring (Colomina et al. 1996; Sánchez et al. 1993; Treinen et al. 1995). On the other hand, Mn deficiency was also related to skeletal malformation and impaired growth in animals (Aschner and Aschner 2005; Hansen et al. 2006). Furthermore, exposure to high levels of Mn is capable of causing oxidative stress in cells and impairing the function and growth of cells (Erikson et al. 2006). Moreover, Mn deficiency has been related to impairments or dysfunctions in lipoprotein metabolism, insulin production, oxidant defense system, and growth factor metabolism (Keen et al. 1999; Tarale et al. 2018). These biological processes caused by either Mn overload or deficiency could be potential mechanisms of Mn-related impairments in fetal development and growth, but more studies are needed to investigate the underlying mechanisms.
Humans are exposed to different metals simultaneously, and the exposure levels of different metals are highly correlated. Additionally, some toxic metals (V, As, Cd, and Pb) have been related to restricted size at birth or increased risk of adverse birth outcomes (Cheng et al. 2017; Hu et al. 2017, 2018; Kippler et al. 2012; Liu et al. 2018; B Zhang et al. 2015). In this study, we observed consistent associations after additionally adjusting for V, As, Cd, and Pb, suggesting that the observed associations between higher levels of Mn during pregnancy and restricted size at birth were not due to confounding by co-exposures to these metals. Moreover, results of sensitivity analyses evaluating the impacts of major confounders (premature deliveries and maternal pregnancy complications) and potential information bias (potential misclassifications caused by missing of urine samples, urine dilution, and urinary Mn concentrations variations) were consistent with the main results, suggesting robust relationships between urinary Mn concentrations and reduced size at birth in the study population.
The main strength of this study was the use of repeated measurements of urinary Mn concentrations in samples that were collected in the first, second, and third trimesters. Additionally, this study was carried out with a large sample size of 3,022 women who were recruited in a Chinese prenatal cohort. Information on outcomes (birth weight, birth length, and ponderal index) and potential confounders (e.g., demographic and socioeconomic characteristics, life-style factors, and gynecological and obstetrical information) was obtained via either medical records or in-person interviews. These advantages provided us the opportunity and enough statistical power to estimate both linear and nonlinear relationships between trimester-specific Mn exposure levels during pregnancy and size at birth. Moreover, because of these strengths, we were able to identify critical windows of susceptibility to Mn exposure during pregnancy that impaired fetal growth (Twisk 2003; Sánchez et al. 2011). Our findings could be generalizable to women in other Chinese cities.
One limitation of this study is that we estimated the Mn exposure levels during pregnancy using urinary concentrations. Mn in the human body is excreted primarily through feces because the human body eliminates Mn mainly through bile (ATSDR 2012). In humans, urinary Mn excretion represents about 5% of the total excreted amount (Smargiassi and Mutti 1999), and the half-life of urinary Mn is less than 30 h (Roels et al. 1987). We used repeatedly measured urinary Mn concentrations in samples collected over different time periods, which may be helpful in reducing the estimation errors caused by one-time measurement. Additional perspective cohort studies with larger sample sizes of women who have repeated measurement of urinary Mn concentrations, as well as studies conducted in other populations, are required to replicate the observed associations in this study. Another limitation of this study was the lack of systemic evaluation of nutritional conditions during pregnancy, which may influence the exposure and outcomes. We, therefore, cannot estimate the contribution and interaction of nutritional conditions during pregnancy on the association between prenatal exposure to Mn and size at birth. Future studies are needed to investigate the effects of mixtures of environmental pollutants (e.g., As, Cd, and Pb) and nutrients (e.g., copper, iron, and zinc) on fetal and childhood growth and development.
Conclusion
In this prospective prenatal cohort study, we observed that prenatal exposure to an elevated level of Mn was associated with significant reductions in birth weight, birth length, and ponderal index. In addition, the late period of pregnancy could be the critical window of heightened vulnerability to Mn exposure for fetal growth. Our findings suggest that high levels of Mn exposure during late pregnancy may be a risk factor for restricted size at birth, and may also be one of the risk factors for childhood and adulthood diseases that have been associated with restricted fetal growth. More studies from other populations and with larger sample sizes are required to replicate these observed associations.
Supplementary Material
Acknowledgments
We thank all the staff and students who made contributions to the cohort study. We thank all the study participants for their support. This work was supported by the National Natural Science Foundation of China (91643207, 91743103, and 21437002), the National Key Research and Development Plan of China (2016YFC0206203 and 2016YFC0206700), and the Fundamental Research Funds for the Central Universities, Huazhong University of Science and Technology (2016YXZD043 and 2018KFYXMPT00).
References
- Agency for Toxic Substances and Disease Registry (ATSDR). 2012. “Toxicological Profile for Manganese.” https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=102&tid=23 [accessed 1 December 2018]. [PubMed]
- Aschner JL, Aschner M. 2005. Nutritional aspects of manganese homeostasis. Mol Aspects Med 26(4–5):353–362, PMID: 16099026, 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. 2007. The origins of the developmental origins theory. J Intern Med 261(5):412–417, PMID: 17444880, 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- Bolze MS, Reeves RD, Lindbeck FE, Kemp SF, Elders MJ. 1985. Influence of manganese on growth, somatomedin and glycosaminoglycan metabolism. J Nutr 115(3):352–358, PMID: 3882911, 10.1093/jn/115.3.352. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Sauvé S, Barbeau B, Legrand M, Brodeur MÉ, Bouffard T. 2011. Intellectual impairment in school-age children exposed to manganese from drinking water. Environ Health Perspect 119(1):138–143, PMID: 20855239, 10.1289/ehp.1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumans PWJM, Ivaldi JC, Slavin W. 1991. Measuring detection limits in inductively coupled plasma emission spectrometry-II. Experimental data and their interpretation. Spectrochim Acta Part B At Spectrosc 46(5):641–665, 10.1016/0584-8547(91)80067-D. [DOI] [Google Scholar]
- Callan AC, Hinwood AL, Ramalingam M, Boyce M, Heyworth J, McCafferty P, et al. . 2013. Maternal exposure to metals—concentrations and predictors of exposure. Environ Res 126:111–117, PMID: 23896418, 10.1016/j.envres.2013.07.004. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2017. “Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, (January 2017).” https://www.cdc.gov/exposurereport/ [acceessed 1 January 2018].
- Chen L, Ding G, Gao Y, Wang P, Shi R, Huang H, et al. . 2014. Manganese concentrations in maternal–infant blood and birth weight. Environ Sci Pollut Res Int 21(9):6170–6175, PMID: 24477335, 10.1007/s11356-013-2465-4. [DOI] [PubMed] [Google Scholar]
- Cheng L, Zhang B, Zheng T, Hu J, Zhou A, Bassig BA, et al. . 2017. Critical windows of prenatal exposure to cadmium and size at birth. Int J Environ Res Public Health 14(1):E59, PMID: 28075368, 10.3390/ijerph14010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KL, Lafayette RA. 2013. Renal physiology of pregnancy. Adv Chronic Kidney Dis 20(3):209–214, PMID: 23928384, 10.1053/j.ackd.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomina MT, Domingo JL, Llobet JM, Corbella J. 1996. Effect of day of exposure on the developmental toxicity of manganese in mice. Vet Hum Toxicol 38(1):7–9, PMID: 8825740. [PubMed] [Google Scholar]
- Crossgrove J, Zheng W. 2004. Manganese toxicity upon overexposure. NMR Biomed 17(8):544–553, PMID: 15617053, 10.1002/nbm.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupul-Uicab LA, Klebanoff MA, Brock JW, Longnecker MP. 2013. Prenatal exposure to persistent organochlorines and childhood obesity in the U.S. collaborative perinatal project. Environ Health Perspect 121(9):1103–1109, PMID: 23799652, 10.1289/ehp.1205901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty SM, Calafat AM, Silva MJ, Ryan L, Hauser R. 2005. Phthalate exposure and reproductive hormones in adult men. Hum Reprod 20(3):604–610, PMID: 15591081, 10.1093/humrep/deh656. [DOI] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). 2013. Scientific opinion on dietary reference values for manganese . EFSA J 11(11):3419, 10.2903/j.efsa.2013.3419. [DOI] [Google Scholar]
- Erikson KM, Dorman DC, Fitsanakis V, Lash LH, Aschner M. 2006. Alterations of oxidative stress biomarkers due to in utero and neonatal exposures of airborne manganese. Biol Trace Elem Res 111(1–3):199–215, PMID: 16943606, 10.1385/BTER:111:1:199. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Thompson K, Aschner J, Aschner M. 2007. Manganese neurotoxicity: a focus on the neonate. Pharmacol Ther 113(2):369–377, PMID: 17084903, 10.1016/j.pharmthera.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eum JH, Cheong HK, Ha EH, Ha M, Kim Y, Hong YC, et al. . 2014. Maternal blood manganese level and birth weight: a MOCEH birth cohort study. Environ Health 13(1):31, PMID: 24775401, 10.1186/1476-069X-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. 2008. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359(1):61–73, PMID: 18596274, 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goullé JP, Mahieu L, Castermant J, Neveu N, Bonneau L, Lainé G, et al. . 2005. Metal and metalloid multi-elementary ICP-MS validation in whole blood, plasma, urine and hair. Reference values. Forensic Sci Int 153(1):39–44, PMID: 15979835, 10.1016/j.forsciint.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Guan H, Wang M, Li X, Piao F, Li Q, Xu L, et al. . 2014. Manganese concentrations in maternal and umbilical cord blood: related to birth size and environmental factors. Eur J Public Health 24(1):150–157, PMID: 23543679, 10.1093/eurpub/ckt033. [DOI] [PubMed] [Google Scholar]
- Gunier RB, Mora AM, Smith D, Arora M, Austin C, Eskenazi B, et al. . 2014. Biomarkers of manganese exposure in pregnant women and children living in an agricultural community in California. Environ Sci Technol 48(24):14695–14702, PMID: 25390650, 10.1021/es503866a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SL, Spears JW, Lloyd KE, Whisnant CS. 2006. Feeding a low manganese diet to heifers during gestation impairs fetal growth and development. J Dairy Sci 89(11):4305–4311, PMID: 17033018, 10.3168/jds.S0022-0302(06)72477-8. [DOI] [PubMed] [Google Scholar]
- He ZL, Yang XE, Stoffella PJ. 2005. Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Biol 19(2–3):125–140, PMID: 16325528, 10.1016/j.jtemb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Health Canada. 2010. “Report on Human Biomonitoring of Environmental Chemicals in Canada: Results of the Canadian Health Measures Survey Cycle 1 (2007–2009).” https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/ewh-semt/alt_formats/hecs-sesc/pdf/pubs/contaminants/chms-ecms/report-rapport-eng.pdf [accessed 1 January 2018].
- Heitland P, Köster HD. 2006. Biomonitoring of 30 trace elements in urine of children and adults by ICP-MS. Clin Chim Acta 365(1–2):310–318, PMID: 16248993, 10.1016/j.cca.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Hu J, Peng Y, Zheng T, Zhang B, Liu W, Wu C, et al. . 2018. Effects of trimester-specific exposure to vanadium on ultrasound measures of fetal growth and birth size: a longitudinal prospective prenatal cohort study. Lancet Planet Health 2(10):e427–e437, PMID: 30318100, 10.1016/S2542-5196(18)30210-9. [DOI] [PubMed] [Google Scholar]
- Hu J, Xia W, Pan X, Zheng T, Zhang B, Zhou A, et al. . 2017. Association of adverse birth outcomes with prenatal exposure to vanadium: a population-based cohort study. Lancet Planet Health 1(6):e230–e241, PMID: 29851608, 10.1016/S2542-5196(17)30094-3. [DOI] [PubMed] [Google Scholar]
- Keen CL, Ensunsa JL, Watson MH, Baly DL, Donovan SM, Monaco MH, et al. . 1999. Nutritional aspects of manganese from experimental studies. Neurotoxicology 20(2–3):213–223, PMID: 10385885. [PubMed] [Google Scholar]
- Kippler M, Wagatsuma Y, Rahman A, Nermell B, Persson LÅ, Raqib R, et al. . 2012. Environmental exposure to arsenic and cadmium during pregnancy and fetal size: a longitudinal study in rural Bangladesh. Reprod Toxicol 34(4):504–511, PMID: 22985739, 10.1016/j.reprotox.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Kirchgessner M, Sherif YS, Schwarz FJ. 1982. Changes in absorption of manganese during gravidity and lactation [in German]. Ann Nutr Metab 26(2):83–89, PMID: 7081955, 10.1159/000176549. [DOI] [PubMed] [Google Scholar]
- Krachler M, Rossipal E, Micetic-Turk D. 1999. Trace element transfer from the mother to the newborn—investigations on triplets of colostrum, maternal and umbilical cord sera. Eur J Clin Nutr 53(6):486–494, PMID: 10403586, 10.1038/sj.ejcn.1600781. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW. 2005. Fetal origins of developmental plasticity: are fetal cues reliable predictors of future nutritional environments? Am J Hum Biol 17(1):5–21, PMID: 15611967, 10.1002/ajhb.20091. [DOI] [PubMed] [Google Scholar]
- Landmann E, Reiss I, Misselwitz B, Gortner L. 2006. Ponderal index for discrimination between symmetric and asymmetric growth restriction: percentiles for neonates from 30 weeks to 43 weeks of gestation. J Matern Fetal Neonatal Med 19(3):157–160, PMID: 16690508, 10.1080/14767050600624786. [DOI] [PubMed] [Google Scholar]
- Liu H, Lu S, Zhang B, Xia W, Liu W, Peng Y, et al. . 2018. Maternal arsenic exposure and birth outcomes: a birth cohort study in Wuhan, China. Environ Pollut 236:817–823, PMID: 29462776, 10.1016/j.envpol.2018.02.012. [DOI] [PubMed] [Google Scholar]
- Ljung KS, Kippler MJ, Goessler W, Grandér GM, Nermell BM, Vahter ME. 2009. Maternal and early life exposure to manganese in rural Bangladesh. Environ Sci Technol 43(7):2595–2601, PMID: 19452922, 10.1021/es803143z. [DOI] [PubMed] [Google Scholar]
- McClatchey KD. 2002. Clinical Laboratory Medicine. 2nd ed Philadelphia, PA:Lippincott Williams & Wilkins. [Google Scholar]
- Menezes-Filho JA, Novaes Cde O, Moreira JC, Sarcinelli PN, Mergler D. 2011. Elevated manganese and cognitive performance in school-aged children and their mothers. Environ Res 111(1):156–163, PMID: 20943219, 10.1016/j.envres.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora AM, van Wendel de Joode B, Mergler D, Córdoba L, Cano C, Quesada R, et al. . 2015. Maternal blood and hair manganese concentrations, fetal growth, and length of gestation in the ISA cohort in Costa Rica. Environ Res 136:47–56, PMID: 25460620, 10.1016/j.envres.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi F, Fukui Y, Takada S, Moriguchi J, Ezaki T, Ikeda M. 2006. Reference values for cobalt, copper, manganese, and nickel in urine among women of the general population in Japan. Int Arch Occup Environ Health 80(2):117–126, PMID: 16736192, 10.1007/s00420-006-0109-4. [DOI] [PubMed] [Google Scholar]
- Peng Y, Hu J, Li Y, Zhang B, Liu W, Li H, et al. . 2018. Exposure to chromium during pregnancy and longitudinally assessed fetal growth: findings from a prospective cohort. Environ Int 121(pt 1):375–382, PMID: 30245360, 10.1016/j.envint.2018.09.003. [DOI] [PubMed] [Google Scholar]
- Rigby RA, Stasinopoulos DM. 2005. Generalized additive models for location, scale and shape. J R Stat Soc Ser C Appl Stat 54:(3):507–554, 10.1111/j.1467-9876.2005.00510.x. [DOI] [Google Scholar]
- Roels H, Lauwerys R, Genet P, Sarhan MJ, de Fays M, Hanotiau I, et al. . 1987. Relationship between external and internal parameters of exposure to manganese in workers from a manganese oxide and salt producing plant. Am J Ind Med 11(3):297–305, PMID: 3578288. [DOI] [PubMed] [Google Scholar]
- Rosner B. 2000. Fundamentals of Biostatistics. 5th ed Pacific Grove, CA:Duxbury. [Google Scholar]
- Sánchez BN, Hu H, Litman HJ, Téllez-Rojo MM. 2011. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect 119(3):409–415, PMID: 21362588, 10.1289/ehp.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez DJ, Domingo JL, Llobet JM, Keen CL. 1993. Maternal and developmental toxicity of manganese in the mouse. Toxicol Lett 69(1):45–52, PMID: 8356567, 10.1016/0378-4274(93)90144-M. [DOI] [PubMed] [Google Scholar]
- Selevan SG, Kimmel CA, Mendola P. 2000. Identifying critical windows of exposure for children’s health. Environ Health Perspect 108(suppl 3):451–455, PMID: 10852844, 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smargiassi A, Mutti A. 1999. Peripheral biomarkers and exposure to manganese. Neurotoxicology 20(2–3):401–406, PMID: 10385899. [PubMed] [Google Scholar]
- Takser L, Lafond J, Bouchard M, St-Amour G, Mergler D. 2004. Manganese levels during pregnancy and at birth: relation to environmental factors and smoking in a Southwest Quebec population. Environ Res 95(2):119–125, PMID: 15147916, 10.1016/j.envres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Tarale P, Daiwile AP, Sivanesan S, Stöger R, Bafana A, Naoghare PK, et al. . 2018. Manganese exposure: linking down-regulation of miRNA-7 and miRNA-433 with α-synuclein overexpression and risk of idiopathic Parkinson’s disease. Toxicol In Vitro 46:94–101, PMID: 28986288, 10.1016/j.tiv.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Tholin K, Palm R, Hallmans G, Sandström B. 1993. Manganese status during pregnancy. Ann N Y Acad Sci 678:359–360, PMID: 8494286, 10.1111/j.1749-6632.1993.tb26146.x. [DOI] [PubMed] [Google Scholar]
- Treinen KA, Gray TJ, Blazak WF. 1995. Developmental toxicity of mangafodipir trisodium and manganese chloride in Sprague-Dawley rats. Teratology 52(2):109–115, PMID: 8588182, 10.1002/tera.1420520207. [DOI] [PubMed] [Google Scholar]
- Tsai MS, Liao KW, Chang CH, Chien LC, Mao IF, Tsai YA, et al. . 2015. The critical fetal stage for maternal manganese exposure. Environ Res 137:215–221, PMID: 25575372, 10.1016/j.envres.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Twisk JWR. 2003. Applied Longitudinal Data Analyses for Epidemiology: A Practical Guide. New York, NY:Cambridge University Press. [Google Scholar]
- Vafeiadi M, Georgiou V, Chalkiadaki G, Rantakokko P, Kiviranta H, Karachaliou M, et al. . 2015. Association of prenatal exposure to persistent organic pollutants with obesity and cardiometabolic traits in early childhood: The Rhea Mother–Child Cohort (Crete, Greece). Environ Health Perspect 123(10):1015–1021, PMID: 25910281, 10.1289/ehp.1409062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RJ. 2009. Manganese and birth outcome. Nutr Rev 67(7):416–420, PMID: 19566601, 10.1111/j.1753-4887.2009.00214.x. [DOI] [PubMed] [Google Scholar]
- Xia W, Zhou Y, Zheng T, Zhang B, Bassig BA, Li Y, et al. . 2016. Maternal urinary manganese and risk of low birth weight: a case–control study. BMC Public Health 16:142, PMID: 26869268, 10.1186/s12889-016-2816-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Xia W, Li Y, Bassig BA, Zhou A, Wang Y, et al. . 2015. Prenatal exposure to lead in relation to risk of preterm low birth weight: a matched case–control study in China. Reprod Toxicol 57:190–195, PMID: 26122562, 10.1016/j.reprotox.2015.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LL, Lu L, Pan YJ, Ding CG, Xu DY, Huang CF, et al. . 2015. Baseline blood levels of manganese, lead, cadmium, copper, and zinc in residents of Beijing suburb. Environ Res 140:10–17, PMID: 25836720, 10.1016/j.envres.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Su X, Su D, Zeng F, Wang MH, Huang L, et al. . 2016. Dietary intake of manganese and the risk of the metabolic syndrome in a Chinese population. Br J Nutr 116(5):853–863, PMID: 27385039, 10.1017/S0007114516002580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Ettinger AS, Bouchard M, Amarasiriwardena CJ, Schwartz J, Hu H, et al. . 2009. Maternal blood manganese levels and infant birth weight. Epidemiology 20(3):367–373, PMID: 19289966, 10.1097/EDE.0b013e31819b93c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.