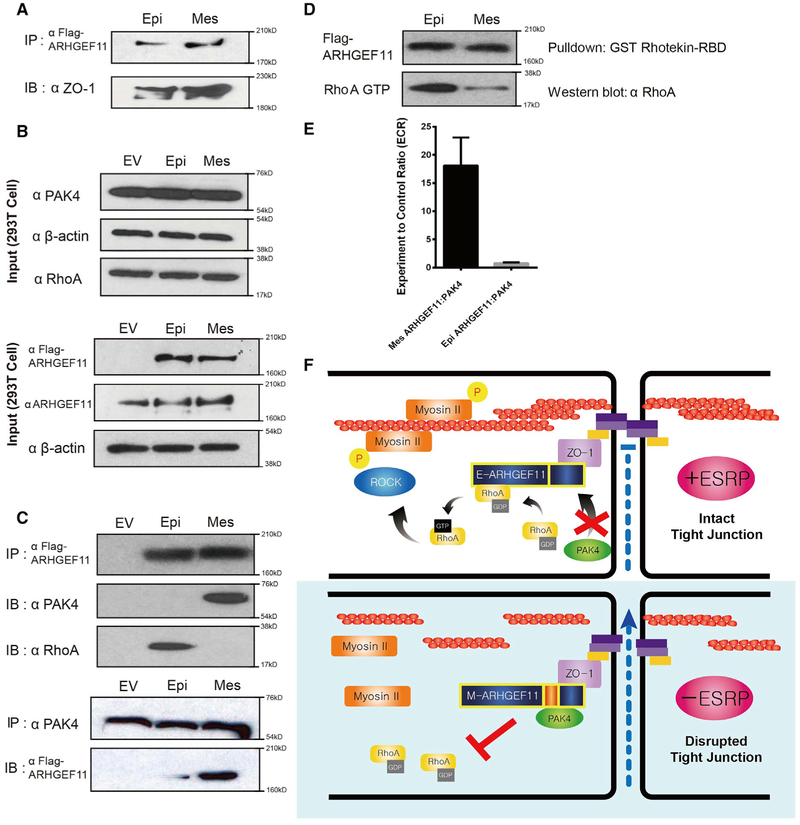
Figure 6. Differential Binding of the Mesenchymal Isoform of ARHGEF11 to Inhibitory PAK4 Leads to Reduced RhoA Activation Compared to the Epithelial Isoform.
(A) FLAG-Arhgef11 isoforms transfected in 293T cells were immunoprecipitated with anti-FLAG antibodies and immunoblotted for ZO-1, demonstrating equivalent ZO-1 binding.
(B) Western immunoblots showing levels of the indicated proteins in input samples.
(C) Transfected FLAG-Arhgef11 isoforms were immunoprecipitated with anti-FLAG antibodies and immunoblotted for p21-activated kinase 4 (PAK4) and RhoA. Immunoprecipitation of endogenous PAK4 confirms preferential binding to the mesenchymal ARHGEF11 isoform.
(D) RhoA activity assay reveals that Epi-Arhgef11 more efficiently activates RhoA than Mes-Arhgef11 when overexpressed in 293T cells.
(E) The mesenchymal but not the epithelial isoform of ARHGEF11 binds to PAK4 in the MAPPIT assay. The experimental-to-control ratio (ECR) is calculated from luciferase readings as outlined in Method Details. Error bar represents SD. Experiments were performed in triplicate.
(F) Schematic of model by which the epithelial isoform of ARHGEF11 maintains the PJAR as a result of reduced binding to inhibitory PAK4.