Figure 2. TGF-β signaling and ECM gene expression are augmented in LMO7 deficient SMCs.
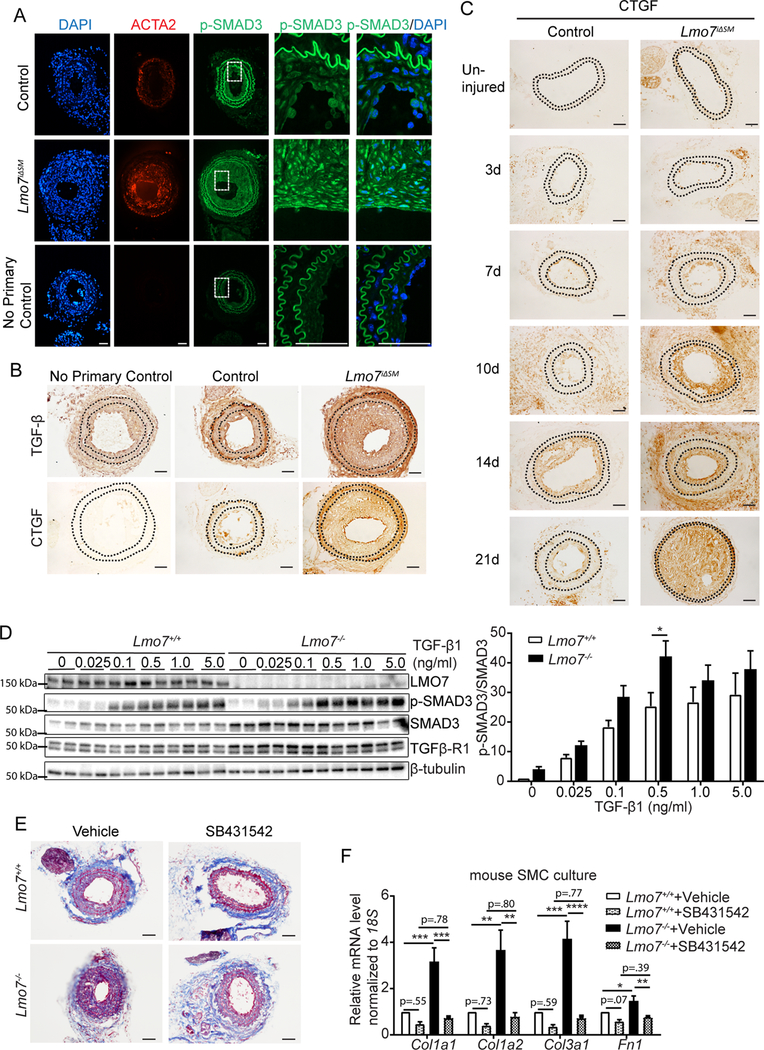
(A, B) Immunostaining of (A) p-SMAD3 (green) or ACTA2 (red) or (B) TGF- β or CTGF in tissue sections from control and Lmo7iΔSM mice after carotid artery ligation. (C) CTGF Immunohistochemistry of arterial sections of control and Lmo7iΔSM mice after carotid artery ligation for durations as indicated. (D) Western analysis of Lmo7+/+ or Lmo7−/− mouse SMCs treated with TGF-β1 at indicated doses for 24 hrs. Quantification of SMAD3 phosphorylation is shown on right (n=6 independent experiments). Two-way ANOVA revealed a significant effect of LMO7 depletion and TGF-β1 dose effect on p-SMAD3 phosphorylation, but there was no significant interaction between Lmo7−/− and TGF-β1 treatment. (E) Lmo7+/+ or Lmo7−/− mice were subjected to carotid ligation and injected intraperitoneally with SB4341542 (10mg/kg/d) or Vehicle (1% DMSO/30% polyethylene/ 1% Tween 80) daily from days 7–28 post-ligation. Trichrome staining of cross-sections of injured arteries is shown. (F) qPCR analysis of ECM genes in Lmo7+/+ or Lmo7−/− mouse SMCs treated with 10 μM SB431542 or Vehicle for 24 hrs (n=5 independent experiments). Two-way ANOVA revealed a significant effect of LMO7 depletion and TGF-β receptor inhibition on mRNA expression. For Col1a1, Col1a2 and Col3a1 mRNA, the magnitude of reduction was greater in Lmo7−/− SMCs (Col1a1 p=0.0057, Col1a2 p=0.019, Col3a1 p=0.0022), indicating LMO7 regulates many ECM genes via TGF-β signaling. Scale bar=50 μm. Data are expressed as mean ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
