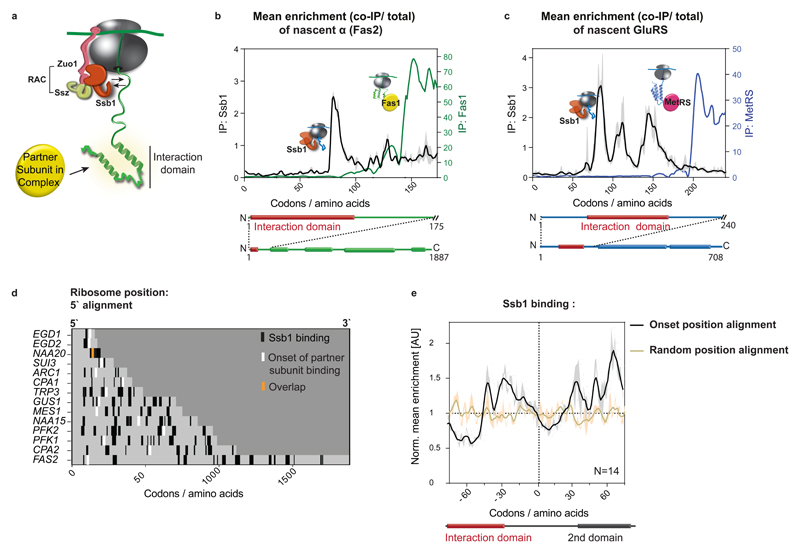
Figure 4. Coordination of cotranslational complex assembly with the ribosome-associated chaperone Ssb binding.
a, Illustration of ribosome–nascent-chain binding to Ssb or a partner subunit. b, c, Zoomed-in interaction profiles of Ssb1–GFP and cotranslationally engaged partner subunits with the nascent FAS α (b, Fas2) and nascent GluRS (c), analysed by SeRP. The area between replicates is shaded, indicating the degree of experimental variation. d, Heat map of Ssb1–GFP binding to ribosomes synthesizing the 14 cotranslationally engaged subunits, compared to complex assembly onset. e, Metagene analysis of Ssb1–GFP interaction profiles with 14 cotranslationally engaged nascent chains, aligned and zoomed-in to assembly onset, compared to random position along the ORFs alignment. There is no correlation between the onset and random position alignment (Pearson correlation r=0.01256), thus Ssb depletion at onset positions is significant. The area between replicates is shaded, indicating the degree of experimental variation. b–e, Data are from two biologically independent experiments.