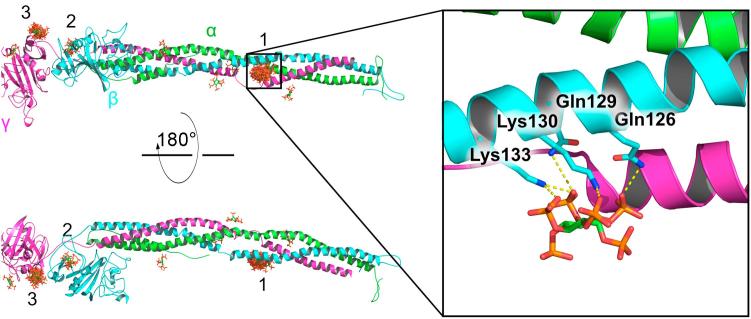
Fig. 4.
Putative InsP6 binding sites at fibrinogen revealed by molecular docking. Fibrinogen is depicted as a cartoon, highlighting the different subunits α, β, and γ by different colors. InsP6 is predicted to bind mainly in three regions (labeled as 1–3). 50 resulting binding poses are overlaid, with InsP6 in stick representation. The region 1, in between the helices of subunits γ and β, clusters the majority of the binding poses (31/50), including the best scored pose and 9 out of the top 15 scored poses. The zoom displays the conformation adopted by InsP6 at this position (for the highest scored pose). Electrostatic interactions are predicted to favor the binding at this site. Potential stabilizing salt bridges and hydrogen bonds interactions between InsP6 and the indicated residues of the β subunit are highlighted by dashed lines.