Abstract
Aims
We compared various components of blood pressure and arterial stiffness of healthy control with those of coronary artery disease (CAD) patients using BP+ machine™.
Methods
In this prospective, case-control study, total 585 individuals of both the genders were enrolled. The study population consisted of 277 controls (healthy siblings of diseased subjects not having CAD – group A) and 308 CAD patients (group B). Age and sex adjusted regression and receiver operative curve (ROC) analysis was performed to assess the strength of association of these parameters.
Results
We found that mean systolic blood pressure (SBP) (137.14 ± 22.49 vs. 129.26 ± 19.86), central systolic blood pressure (CSBP) (130.78 ± 21.89 vs. 117.53 ± 17.98), augmentation index (AI) (108.55 ± 44.98 vs. 49.38 ± 21.03) and pulse rate variability (98.82 ± 231.09 vs. 82.86 ± 208.77) were significantly (p < 0.05) higher in CAD population as compared to healthy counterparts. Left ventricular contractibility as measured by dP/dt was significantly lower in CAD patients. All these parameters were significantly abnormal in CAD as compared to healthy control population irrespective of the gender of the patient except for SBP in females. Both – odds ratio (1.108; 95% CI: 1.081–1.135; p < 0.0001) and ROC analysis (AUC: 0.937; 95% CI: 0.919–0.956; p < 0.0001) showed AI as the strongest predictor of CAD, closely followed by CSBP.
Conclusion
Central aortic blood pressure parameters such as AI and CSBP measured noninvasively with BP+ machine could be the effective predictors of CAD in Asian Indians.
Keywords: Coronary artery disease, Central blood pressure, Augmentation index, Asian Indians
1. Introduction
Cardiovascular diseases (CVD) including coronary artery disease (CAD) and stroke, are leading cause of mortality and morbidity especially in the developing countries of the world.1 Asian Indians exhibits unique phenotypic characteristics that distinguishes them from the other population phenotype, suggesting a possible involvement of unique causative factors.2 Prognostication and predictability of CAD and other cardiovascular events is multifactorial in nature involving interactions between genetic and environmental factors over an extended period of time.
The identification of measurable “risk factors” in the community at large may provide insight into the prognostication and early diagnosis of the disease.18 The compliance of the arteries in the human body tends to decline with age, and this process starts from early childhood even in the absence of established cardiovascular diseases.3, 4, 5 The vascular compliance is reduced in hypertensive subjects, patients with end-stage renal disease and those with diabetes mellitus.6 Increased arterial stiffness per se is an independent marker of cardiovascular disease in hypertensive subjects and is linked to ventricular hypertrophy and atherosclerosis in certain studies.7 The association between elevated blood pressure, atherosclerosis, and major cardiovascular events has also been extensively documented.8 The brachial artery sphygmomanometry is as a time tested and often the standard method for determining systolic blood pressure (SBP) and diastolic blood pressure (DBP), where cuff measurements of blood pressure are recommended for the diagnosis of arterial hypertension and the assessment of cardiovascular risk. SBP and pulse pressure (PP) have been, associated with the occurrence of cardiovascular events in the long term and in some studies more than other blood pressure parameters.9, 10, 11, 12, 13
Central blood pressure (CBP) is the pressure measured in the central aorta that is often different from the pressure measured in the peripheral artery of arm. Emerging reports suggest that CBP is a more reliable marker than peripherally measured pressures for the pathogenesis of cardiovascular disease and hence is strongly related to future cardiovascular events rather than the brachial pressure measured peripherally.14, 15 In spite of the available non-invasive methods of CBP monitoring, it’s practical use in risk stratification of CVD patients and for effective monitoring of treatment strategies by clinicians is less accessible and hence less popular as compared to peripheral pressure. “Augmentation” which reflects enhancement of central aortic pressure is commonly expressed as Augmentation Index (AI), which is also considered as a sensitive marker of arterial wall condition and stiffness. However clinical worthiness of AI still remains less well established.16, 17
In one of the studies, AI significantly correlated with GENSINI and SYNTAX scores in CAD patients who underwent coronary angiography even after adjusting for age, gender, height, heart rate, hypertension, and diabetes.35
We here with aimed to compare and evaluate the predictive potential of isolated SBP, DBP, central systolic blood pressure (CSBP), central diastolic blood pressure (CDBP), AI, maximum rate of pressure rise (dP/dt max), heart rate and other parameters measured noninvasively by blood pressure measurement using BP+ machine™ by USCOM (sphygmomanometer machine) that estimates the central parameters based on the waveform analysis in CAD patients and compared the same with healthy Indians. We also sought to assess the influence of age, gender and other confounding factors on predictive value of these measurable parameters of vascular stiffness in patients with CAD as compared to healthy controls.
2. Material and methods
2.1. Design and data collection
In this case control study, a total 585 individuals of both the genders (411 males and 174 females), ranging in age from 30 to 80 years, who visited the cardiac clinic and were enrolled. The study was approved and cleared by an independent Ethics Committee. Out of 585 subjects, 277 were controls (healthy siblings of diseased subjects not having CAD) (group A), and 308 (group B) were established CAD patients who were on stable medication for last 3 months. The demographic and risk factor details of the study population were collected. All the parameters including vascular age were captured as measured by the BP+ machineTM used in the study.
2.2. Blood pressure measurements
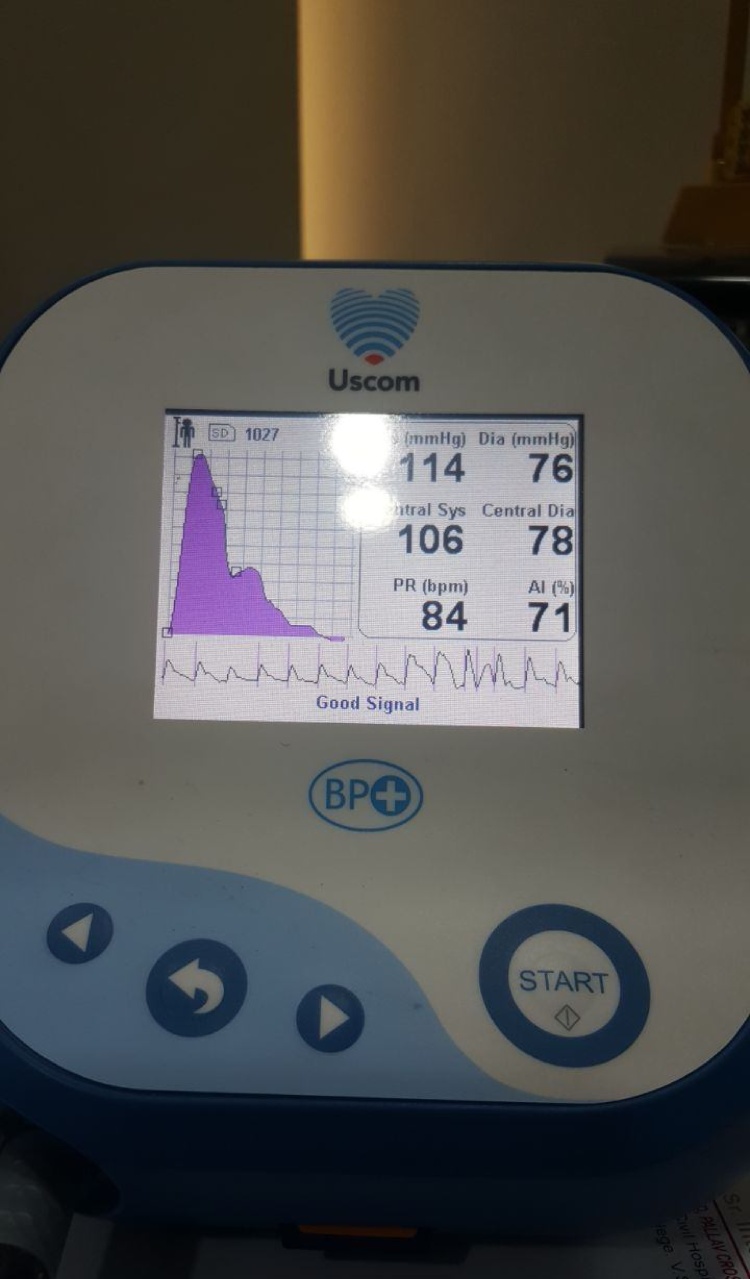
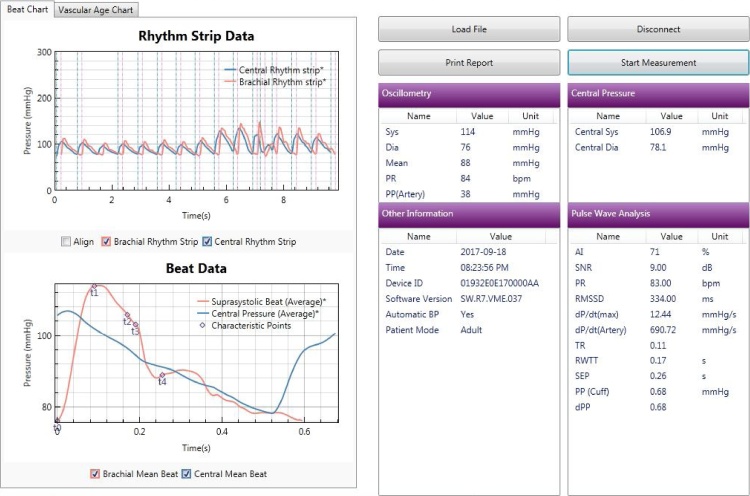
Non-invasive brachial BP was measured using a “BP+ Central Blood Pressure machine by USCOM”. The BP+ is an apparatus that measures central and peripheral BP, arterial stiffness, AI, pulse rate variability using suprasystolic oscillometry and performs central and peripheral pulse waveform analysis. The BP+ by USCOM is patented machine (Fig. 1) and is commercially available for assessment of these parameters using the waveform analysis as shown in Fig. 2 and estimates vascular-age from the age and sex specified normograms as shown in Fig. 3. The machine measures pAI and that has been used as the parameter in this study.
Fig. 1.
BP+ machine from USCOM.
Fig. 2.
Methodology of Pulse Wave analysis with measurement of AI.
Fig. 3.
Estimation of Vascular age from AI normograms.
Central blood pressure was calculated using a physics-based model of the arteries between the aorta and the cuff. This model relates to how the pressure waves travel between the aorta and the occluded artery under the suprasystolic cuff. This instrument also calculates CSBP, CDBP and mean pressures.
Mean dP/dt was calculated using the following equation:
| [(aortic diastolic pressure) − (ventricular end-diastolic pressure)]/isovolumetric contraction time |
Parameters were measured in the right arm of the subject, in the supine position. The mean of the three readings was used as the blood pressure of the subjects. All the parameters were recorded immediately before tonometric recording.
2.3. Parameters of arterial stiffness measures
| Augmentation Index (AI) (%): It can be expressed as central or peripheral AI. |
Central augmentation index (cAI) is defined for a central pressure waveform as a ratio of augmentation pressure (AP) to pulse pressure (PP):
-
•
cAI = AP/PP × 100
The shape of the wave in central arteries is different for a pressure waveform obtained at the upper arm or wrist. In some cases, late systolic pressure may be higher than early systolic pressure. In other cases, there may be no visible pressure augmentation (late systolic pressure is lower than early systolic pressure).
-
•
Peripheral augmentation index = pAI = S1/s2 and is derived from the waveforms.
2.4. Statistical analysis
The statistical calculations were performed using SPSS software v 20.0 (Chicago, IL, USA) Quantitative data was expressed as mean ± SD, whereas qualitative data was expressed as percentage. Univariate analysis of the continuous data was performed using student’s t-test or Mann Whitney U test, whichever was applicable. The cut off value of p < 0.05 was considered for statistical significance. Age and sex – adjusted linear regression model was applied to the data to measure the strength of a particular parameter in predicting CAD. Receiver operating curve (ROC) analysis was performed to assess the diagnostic accuracy of various parameters.
3. Results
The comparison of various risk factors between cases and controls is presented in (Table 1). Mean age of group B patients was significantly lower as compared to group A subjects. The prevalence of CAD risk factors was higher in CAD patients as compared to healthy controls (diabetes: 12% vs. 5%, hypertension: 45% vs. 15%, smoking habit: 13% vs. 6%; dyslipidemia: 51% vs. 30%; obesity: 6% vs. 4.2%). The mean SBP (137.14 ± 22.49 vs. 129.26 ± 19.86), CSBP (130.78 ± 21.89 vs. 117.53 ± 17.98) and AI% (108.55 ± 44.98 vs. 49.38 ± 21.03) were significantly (p < 0.05) higher in CAD patients as compared to healthy population. Amongst SBP, CSBP and AI, the difference in AI% had the strongest coefficient of correlation between these two study groups. Patients with CAD had statistically higher vascular age and pulse rate variability as compared to their healthy controls. Dp/dt reflects the rate of increase in pressure in the artery per unit time hence reflecting a measure of left ventricular function and contractility. It was also significantly (870.03 ± 335.39 vs 963.18 ± 304.63; p < 0.0001) lower in group B as compared to group A.
Table 1.
Comparison of risk factor distribution in healthy vs control population.
| Variables | Groups | Overall population |
Male population |
Female population |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Standard Deviation | Significance | Mean | Standard Deviation | Significance | Mean | Standard Deviation | Significance | ||
| Age | Group A | 57.05 | 11.84 | <0.0001 | 57.53 | 11.02 | <0.0001 | 56.06 | 13.37 | <0.0001 |
| Group B | 50.87 | 11.48 | 51.58 | 11.22 | 48.93 | 12.03 | ||||
| SBP | Group A | 129.25 | 19.86 | <0.0001 | 127.39 | 19.08 | <0.0001 | 133.08 | 21.08 | 0.085 |
| Group B | 137.13 | 22.48 | 136.38 | 21.42 | 139.18 | 25.17 | ||||
| DBP | Group A | 75.51 | 13.53 | 0.086 | 75.09 | 13.58 | 0.102 | 76.34 | 13.53 | 0.468 |
| Group B | 77.53 | 14.61 | 77.4 | 14.77 | 77.87 | 14.22 | ||||
| CSBP | Group A | 117.53 | 17.98 | <0.0001 | 115.73 | 17.54 | <0.0001 | 121.24 | 18.4 | <0.0001 |
| Group B | 130.77 | 21.89 | 130.03 | 21.2 | 132.8 | 23.68 | ||||
| CDBP | Group A | 78.71 | 13.73 | 0.107 | 78.13 | 13.8 | 0.068 | 79.92 | 13.59 | 0.842 |
| Group B | 80.65 | 15.05 | 80.75 | 15.03 | 80.35 | 15.2 | ||||
| AI (%) | Group A | 49.38 | 21.02 | <0.0001 | 47.79 | 20.65 | <0.0001 | 52.66 | 21.52 | <0.0001 |
| Group B | 108.55 | 44.98 | 108.16 | 45.6 | 109.62 | 43.52 | ||||
| Vascular age | Group A | 44.38 | 15.99 | <0.0001 | 43.83 | 16 | <0.0001 | 45.51 | 15.98 | <0.0001 |
| Group B | 83.2 | 18.51 | 83.67 | 18.21 | 81.93 | 19.34 | ||||
| Difference between chronological and vascular age | Group A | −13.58 | 17.6 | <0.0001 | −14.4 | 17.55 | <0.0001 | −12.05 | 17.72 | <0.0001 |
| Group B | 32.33 | 17.1 | 32.08 | 16.37 | 33 | 19.04 | ||||
| PP | Group A | 0.71 | 0.3 | 0.502 | 0.7 | 0.29 | 0.744 | 0.73 | 0.32 | 0.488 |
| Group B | 0.73 | 0.41 | 0.74 | 0.45 | 0.71 | 0.3 | ||||
| Pulse Rate Variability | Group A | 82.86 | 208.77 | 0.0001 | 81.41 | 203.85 | 0.004 | 85.87 | 219.72 | 0.041 |
| Group B | 98.82 | 231.09 | 109.13 | 257.05 | 70.87 | 135.48 | ||||
| dP/dt artery | Group A | 963.18 | 304.62 | <0.0001 | 937.09 | 286.24 | 0.013 | 1017.09 | 334.75 | 0.019 |
| Group B | 870.03 | 335.38 | 862.15 | 324.77 | 891.4 | 363.82 | ||||
AI = Augmentation index, CAD = Coronary artery disease, CDBP = Central diastolic blood pressure, CSBP = Central systolic blood pressure, DBP = Diastolic blood pressure, SBP = Systolic blood pressure, PP = Pulse pressure.
The risk factor profile in male and female is presented in (Table 1) respectively. In males, significant (p < 0.05) association of CAD was observed with SBP, CSBP, AI%, pulse rate variability and dp/dt, whereas in females it was significant for CSBP, AI%, pulse rate variability and dp/dt but not for SBP. The overall predictive value of risk factors for CAD is displayed in (Table 2). AI as measured by BP+ machineTM emerged as the strongest predictor (Odds ratio: 1.108; 95% CI: 1.081–1.135; p < 0.0001) of CAD in this study. AI had the highest odds ratio amongst both the gender. (Male – odds ratio: 1.102; 95% CI: 1.080–1.124; p < 0.0001/Female −odds ratio: 1.094; 95% CI: 1.063–1.125; p < 0.0001) (Table 3, Table 4).The ROC analysis showed that in the study population irrespective of the gender of the population, AI had the highest AUC, and was closely followed by CSBP (Table 5).
Table 2.
Regression analysis in overall population.
| Variables | B | S.E. | Wald | df | Significance | Exp(B) | 95% C.I.for EXP(B) |
|
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| SBP | −0.024 | 0.05 | 0.224 | 1 | 0.636 | 0.977 | 0.886 | 1.077 |
| CSBP | 0.027 | 0.051 | 0.278 | 1 | 0.598 | 1.027 | 0.93 | 1.134 |
| AI (%) | 0.102 | 0.012 | 67.146 | 1 | 0 | 1.108 | 1.081 | 1.135 |
| Pulse Rate Variability | 0.001 | 0.001 | 0.529 | 1 | 0.467 | 1.000 | 0.999 | 1.002 |
| dP/dt artery | 0.001 | 0.001 | 0.616 | 1 | 0.433 | 1.001 | 0.999 | 1.002 |
| Constant | −7.859 | 1.445 | 29.584 | 1 | 0 | 0 | ||
AI = Augmentation index, CSBP = Central systolic blood pressure, SBP = Systolic blood pressure.
Table 3.
Regression analysis in male population.
| Variables | B | S.E. | Wald | df | Significance | Exp(B) | 95% C.I.for EXP(B) |
|
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| SBP | −0.022 | 0.038 | 0.325 | 1 | 0.569 | 0.978 | 0.907 | 1.055 |
| CSBP | 0.022 | 0.04 | 0.322 | 1 | 0.571 | 1.023 | 0.946 | 1.105 |
| AI (%) | 0.097 | 0.01 | 93.955 | 1 | 0 | 1.102 | 1.08 | 1.124 |
| Pulse rate variability | 0.001 | 0.001 | 2.164 | 1 | 0.141 | 1.001 | 1.000 | 1.003 |
| dP/dt artery | 0 | 0.001 | 0.68 | 1 | 0.41 | 1 | 0.999 | 1.002 |
| Constant | −7.269 | 1.097 | 43.909 | 1 | 0 | 0.001 | ||
AI = Augmentation index, CSBP = Central systolic blood pressure, SBP = Systolic blood pressure.
Table 4.
Regression analysis in female population.
| Variables | B | S.E. | Wald | df | Significance | Exp(B) | 95% C.I.for EXP(B) |
|
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| CSBP | 0 | 0.015 | 0 | 1 | 0.983 | 1 | 0.971 | 1.03 |
| AI (%) | 0.089 | 0.015 | 37.153 | 1 | 0 | 1.094 | 1.063 | 1.125 |
| Pulse rate variability | 0 | 0.001 | 0.97 | 1 | 0.755 | 1.000 | 0.998 | 1.002 |
| dP/dt artery | 0 | 0.001 | 0.051 | 1 | 0.822 | 1 | 0.999 | 1.002 |
| Constant | −7.011 | 1.663 | 17.771 | 1 | 0 | 0.001 | ||
AI = Augmentation index, CSBP = Central systolic blood pressure.
Table 5.
Receiver operative curve analysis in overall, male and female population.
| Area Under the Curve | |||||
|---|---|---|---|---|---|
| Overall | |||||
| Variables | Area | Standard Deviation | Asymptotic Sig. | Asymptotic 95% Confidence Interval |
|
| Lower Bound | Upper Bound | ||||
| SBP | 0.598 | 0.023 | 0.000 | 0.552 | 0.644 |
| CSBP | 0.671 | 0.022 | 0.000 | 0.628 | 0.714 |
| AI (%) | 0.937 | 0.009 | 0.000 | 0.919 | .956 |
| Pulse rate variability | 0.585 | 0.024 | 0.000 | 0.538 | 0.631 |
| dP/dt (artery) | 0.399 | 0.023 | 0.000 | 0.353 | 0.445 |
| Males | |||||
| SBP | 0.614 | 0.028 | 0.000 | 0.560 | 0.669 |
| CSBP | 0.686 | 0.026 | 0.000 | 0.635 | 0.737 |
| AI (%) | 0.946 | 0.010 | 0.000 | 0.926 | 0.966 |
| Pulse rate variability | 0.583 | 0.028 | 0.004 | 0.527 | 0.639 |
| dP/dt (artery) | 0.419 | 0.028 | 0.005 | 0.364 | 0.474 |
| Females | |||||
| CSBP | 0.645 | 0.042 | 0.001 | 0.563 | 0.727 |
| AI (%) | 0.918 | 0.021 | 0.000 | 0.877 | 0.959 |
| Pulse rate variability | 0.590 | 0.043 | 0.041 | 0.505 | 0.675 |
| dP/dt (artery) | 0.357 | 0.043 | 0.001 | 0.274 | 0.441 |
AI = Augmentation index, CSBP = Central systolic blood pressure, SBP = Systolic blood pressure.
4. Discussion
Augmentation index – a marker of arterial wave reflection, is proposed to indicate cardiovascular risk burden that can be assessed noninvasively. Although it is universally accepted as clinical index of arterial stiffness, its practical use for prediction of CAD is debatable as a few studies have reported lack of association between AI and CAD especially in the elderly population.19Current study clearly demonstrates an independent relationship between AI and CAD in Asian Indians and recommends it as one of the strongest predictor of CAD in Asian Indians.
The proposal of AI as novel CAD index is of importance as conventionally assessed branchial artery blood pressure often ill estimates the actual central pressure. Difference between aortic and branchial systolic pressure could be as high as 20 mmHg due to pressure wave amplification often undermining central-peripheral disparity in terms of perfusion and hypertension.20, 21, 22 AI is known to be influenced by age, gender and height of an individual,23, 24, 25 however; in current study, AI was found to be an independent predictor of CAD on regression and ROC analysis. This study also reports that predictive value of AI is least affected by gender and it predicts the CAD risk with equal strength in both the genders.
Earlier studies had indicated that indices derived from central blood pressure measurement are different and independent from traditional cuff blood pressure measurements with regard to their prognostic value for CAD.26, 27, 28, 29 AI is particularly suited to unmask atherosclerosis in young and probably elderly people as well. In concordance with this, few more studies have shown a significant correlation between the elevated central aortic AI and an increased risk of CAD.30, 31 According to one of the recent study, increased AI is associated with the presence and severity of CAD, and it could be effectively be used to risk stratify the patients prior to coronary angiography.32, 33, 34, 35
Systolic blood pressure has consistently shown a consistent linear relationship with cardiovascular morbidity and mortality in all age groups and in both the genders.33.34 However the levels of DBP could remain normal even in presence of cardiovascular risk as increased peripheral vascular resistance combined with increased arterial stiffening may nullify the effect of each other leading to depiction of normal DBP levels. DBP might have lesser prognostic value in older patients, where the prevailing form of hypertension could be an isolated systolic hypertension. As arterial stiffening causes SBP to increase and DBP to decrease, in such population PP may be the best predictor of cardiovascular events across all blood pressure values. With increasing age among participants in the Framingham study, there was a gradual shift from DBP to SBP and then to PP as a predictor of coronary risk. This has led some commentators to suggest that PP should become part of the Framingham risk algorithm. However, we herewith report that in older population also AI seems to provide remarkable predictive potential, closely followed by CSBP.
5. Conclusion
The results of this study corroborate the significance of the AI as a sensitive predictor of CAD. Augmentation index, either taken at a single measurement, or along with CSBP, could identify patients with CAD in Asian Indian population. This simple tool of BP measurement when combined with AI can help in prognostication and early diagnosis of CAD in this ethnic group, especially in females where SBP may not be very different from control population as elucidated by this study.
Conflict of interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Celermajer D.S., Chow C.K., Marijon E., Anstey N.M., Woo K.S. Cardiovascular disease in the developing world: prevalence’s, patterns, and the potential of early disease detection. J Am Coll Cardiol. 2012;60:1207–1216. doi: 10.1016/j.jacc.2012.03.074. [DOI] [PubMed] [Google Scholar]
- 2.Gupta M., Brister S., Verma S. Is South Asian ethnicity an independent cardiovascular risk factor. Can J Cardiol. 2006;22:193–197. doi: 10.1016/s0828-282x(06)70895-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gozna E.R., Marble A.E., Shaw A., Holland J.G. Age related changes in the mechanics of the aorta and pulmonary artery of man. J Appl Physiol. 1974;36:407–411. doi: 10.1152/jappl.1974.36.4.407. [DOI] [PubMed] [Google Scholar]
- 4.Bulpitt C.J., Cameron J.D., Rajkumar C. The effect of age on vascular compliance in man. Which are the appropriate measures? J Hum Hypertens. 1999;13:753–758. doi: 10.1038/sj.jhh.1000879. [DOI] [PubMed] [Google Scholar]
- 5.Laogun A.A., Goslin R.G. In vivo arterial compliance in man. Clin Phys Physiol Meas. 1982;3:201–212. doi: 10.1088/0143-0815/3/3/004. [DOI] [PubMed] [Google Scholar]
- 6.Theilade S., Lajer M., Persson F., Joergensen C., Rossing P. Arterial stiffness is associated with cardiovascular, renal, retinal, and autonomic disease in type 1 diabetes. Diabetes Care. 2013;36:715–721. doi: 10.2337/dc12-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blacher J., Asmar R., Djane S., London G.M., Safar M.E. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–1117. doi: 10.1161/01.hyp.33.5.1111. [DOI] [PubMed] [Google Scholar]
- 8.Hollander W. Role of hypertension in atherosclerosis and cardiovascular disease. Am J Cardiol. 1976;38:786–800. doi: 10.1016/0002-9149(76)90357-x. [DOI] [PubMed] [Google Scholar]
- 9.Sesso H.D., Stampfer M.J., Rosner B. Systolic and diastolic blood pressure, pulse pressure, and mean arterial pressure as predictors of cardiovascular disease risk in Men. Hypertension. 2000;36:801–807. doi: 10.1161/01.hyp.36.5.801. [DOI] [PubMed] [Google Scholar]
- 10.Domanski M.J., Davis B.R., Pfeffer M.A., Kastantin M., Mitchell G.F. Isolated systolic hypertension: prognostic information provided by pulse pressure. Hypertension. 1999;34:375–380. doi: 10.1161/01.hyp.34.3.375. [DOI] [PubMed] [Google Scholar]
- 11.Franklin S.S., Khan S.A., Wong N.D., Larson M.G., Levy D. Is pulse pressure useful in predicting risk for coronary heart disease: the Framingham heart study. Circulation. 1999;100:354–360. doi: 10.1161/01.cir.100.4.354. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell G.F., Moyé L.A., Braunwald E. Sphygmomanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. SAVE investigators. Survival and ventricular enlargement. Circulation. 1997;96:4254–4260. doi: 10.1161/01.cir.96.12.4254. [DOI] [PubMed] [Google Scholar]
- 13.Franklin S.S., Lopez V.A., Wong N.D. Single versus combined blood pressure components and risk for cardiovascular disease: the Framingham Heart Study. Circulation. 2009;119:243–250. doi: 10.1161/CIRCULATIONAHA.108.797936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safar M.E., Blacher J., Pannier B. Central pulse pressure and mortality in end-stage renal disease. Hypertension. 2002;39:735–738. doi: 10.1161/hy0202.098325. [DOI] [PubMed] [Google Scholar]
- 15.Roman M.J., Devereux R.B., Kizer J.R. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50:197–203. doi: 10.1161/HYPERTENSIONAHA.107.089078. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu M., Kario K. Review: role of the augmentation index in hypertension. Ther Adv Cardiovasc Dis. 2008;2:25–35. doi: 10.1177/1753944707086935. [DOI] [PubMed] [Google Scholar]
- 17.Townsend R.R., Wilkinson I.B., Schiffrin E.L. on behalf of the American Heart Association Council on Hypertension: recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Agostino R.B., Vasan R.S., Pencina M.J. General cardiovascular risk profile for use in primary care the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi S., Yamada H., Bando M. Augmentation index does not reflect risk of coronary artery disease in elderly patients. Circ J. 2014;78:1176–1182. doi: 10.1253/circj.cj-13-1422. [DOI] [PubMed] [Google Scholar]
- 20.Nichols W.W., O'Rourke M.F. 5th ed. Hodder Arnold; London: 2005. McDonald's blood flow in arteries: theoretical, experimental and clinical principles. Vascular impedance; pp. 216–231. [Google Scholar]
- 21.Protogerou A.D., Papaioannou T.G., Blacher J., Papamichael C.M., Lekakis J.P., Safar M.E. Central blood pressures: do we need them in the management of cardiovascular disease? Is it a feasible therapeutic target? J Hypertens. 2007;25:265–272. doi: 10.1097/HJH.0b013e3280114f23. [DOI] [PubMed] [Google Scholar]
- 22.Pauca A.L., Wallenhaupt S.L., Kon N.D., Tucker W.Y. Does radial artery pressure accurately reflect aortic pressure. Chest. 1992;102:1193–1198. doi: 10.1378/chest.102.4.1193. [DOI] [PubMed] [Google Scholar]
- 23.Kelly R., Hayward C., Avolio A., O'Rourke M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation. 1989;80:1652–1659. doi: 10.1161/01.cir.80.6.1652. [DOI] [PubMed] [Google Scholar]
- 24.Hayward C.S., Kelly R.P. Gender-related differences in the central arterial pressure waveform. J Am Coll Cardiol. 1997;30:1863–1871. doi: 10.1016/s0735-1097(97)00378-1. [DOI] [PubMed] [Google Scholar]
- 25.McGrath B.P., Liang Y.L., Kotsopoulos D., Cameron J.D. Impact of physical and physiological factors on arterial function. Clin Exp Pharmacol Physiol. 2001;28:1104–1107. doi: 10.1046/j.1440-1681.2001.03591.x. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi T., Nakayama Y., Tsumura K., Yoshimaru K., Ueda H. Reflection in the arterial system and the risk of coronary heart disease. Am J Hypertens. 2002;15:405–409. doi: 10.1016/s0895-7061(02)02260-4. [DOI] [PubMed] [Google Scholar]
- 27.Jankowski P., Kawecka-Jaszcz K., Bryniarski L. Fractional diastolic and systolic pressure in the ascending aorta are related to the extent of coronary artery disease. Am J Hypertens. 2004;17:641–646. doi: 10.1016/j.amjhyper.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Weber T., Auer J., O'Rourke M.F. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109:184–189. doi: 10.1161/01.CIR.0000105767.94169.E3. [DOI] [PubMed] [Google Scholar]
- 29.Chirinos J.A., Zambrano J.P., Chakko S. Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease. Hypertension. 2005;45:980–985. doi: 10.1161/01.HYP.0000165025.16381.44. [DOI] [PubMed] [Google Scholar]
- 30.Weber T., Auer J., O'rourke M.F. Increased arterial wave reflections predict severe cardiovascular events in patients undergoing percutaneous coronary interventions. Eur Heart J. 2005;26:2657–2663. doi: 10.1093/eurheartj/ehi504. [DOI] [PubMed] [Google Scholar]
- 31.Covic A., Haydar A.A., Bhamra-Ariza P., Gusbeth-Tatomir P., Goldsmith D.J. Aortic pulse wave velocity and arterial wave reflections predict the extent and severity of coronary artery disease in chronic kidney disease patients. J Nephrol. 2005;18:388–396. [PubMed] [Google Scholar]
- 32.‘Tanindi A., Erkan A.F., Alhan A., Töre H.F. Central pulse pressure amplification is associated with more extensive and severe coronary artery disease. Scand Cardiovasc J. 2014;48:167–175. doi: 10.3109/14017431.2014.898083. [DOI] [PubMed] [Google Scholar]
- 33.Kannel W.B., D'Agostino R.B., Silbershatz H. Blood pressure and cardiovascular morbidity and mortality rates in the elderly. Am Heart J. 1997;134:758–763. doi: 10.1016/s0002-8703(97)70061-9. [DOI] [PubMed] [Google Scholar]
- 34.Weber T., Auer J., O'rourke M.F. Blood pressure and cardiovascular morbidity and mortality in a Dutch population: the Nijmegen cohort study. Prev Med. 2001;32:142–147. doi: 10.1006/pmed.2000.0764. [DOI] [PubMed] [Google Scholar]
- 35.Tanindi A., Erkan A.F., Alhan A., Töre H.F. Central pulse pressure amplification is associated with more extensive and severe coronary artery disease. Scand Cardiovasc J. 2014;48(January (3)):167–175. doi: 10.3109/14017431.2014.898083. [DOI] [PubMed] [Google Scholar]