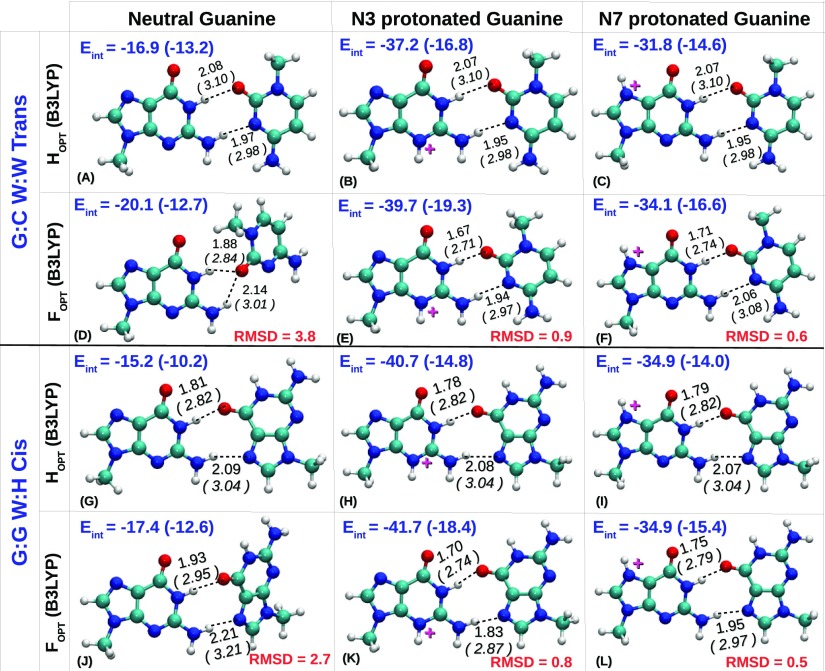
Figure 3.
HOPT and FOPT geometries of G:C W:W Trans (A−F) and G:G W:H Cis (G−L) base pairs obtained at B3LYP/6-31+G(d,p). The optimizations have been performed with three different protonation states of guanine: neutral, N3-protonated, and N7-protonated. Interbase hydrogen bonds are shown in broken lines. For each hydrogen bond, the distance between hydrogen and acceptor atom (in normal font) and the distance between the donor and acceptor atom (in italic font, within parentheses) are reported in Å. Interaction energies (Eint) of the base pairs (calculated at MP2/aug-cc-pVDZ) have been reported in kcal mol–1. The values in parenthesis represent the solvent-phase interaction energies. RMSD values between the FOPT and HOPT geometries are reported in Å.