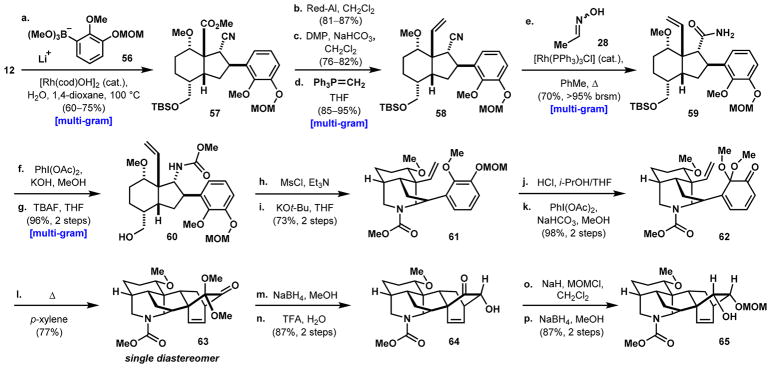
Scheme 8. Formation of the Key Hexacyclic Intermediate Containing the [2.2.2] Bicyclea.
aReaction conditions: (a) 56 (prepared from the corresponding ArBr), n-BuLi, Et2O, –78 to 23 °C; then B(OMe)3, –40 °C; then 12, [Rh(cod)OH]2 (cat.), H2O, 1,4-dioxane, 100 °C, 60–75%; (b) Red-Al, CH2Cl2, 0–23 °C, 81–87%; (c) DMP, NaHCO3, CH2Cl2, 76–82%; (d) PPh3MeBr, LiHMDS, THF, 70 °C; then aldehyde, 0–23 °C, 85–95%; (e) 28, [Rh(PPh3)3Cl] (cat), PhMe, 130 °C, 70% (>95% brsm); (f) PhI(OAc)2, KOH, MeOH; (g) TBAF, THF, 23 °C, 96% over 2 steps; (h) MsCl, Et3N, CH2Cl2; (i) KOt-Bu, THF, 73% over two steps; (j) HCl, i-PrOH/THF; (k) PhI(OAc)2, NaHCO3, MeOH, 98% over two steps; (l) p-xylene, 150 °C, 77%; (m) NaBH4, MeOH; (n) TFA, H2O, 87% over two steps; (o) NaH, MOMCl, CH2Cl2; (p) NaBH4, MeOH, 87% over 2 steps.