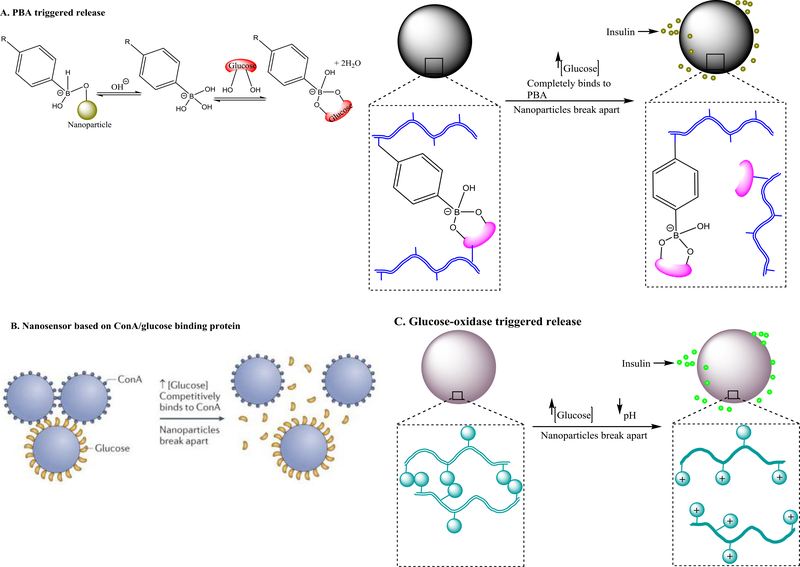
Figure 7.
Design strategies for glucose-responsive nanotherapeutics and mechanism of sensing and/or drug release. (a) Phenylboronic acid (PBA)-triggered glucose sensing and insulin release. Upon glucose exposure in aqueous milieu, the charged form of PBA attached to nanoparticles forms complexation with glucose by hydrogen binding, shifting the equilibrium to the direction of producing more hydrophilic forms of PBA to form complexation with glucose. Owing to the complexation, the nanoparticles undergo volumetric and phase change from hydrophillic to hydrophobic to release the loaded inuslin. The complexation between the PBA and glucose can also be preformed by conjuating them on different polymer chains of the nanoparticles before exposure to high glucose conditions. Under hyperglycemic conditions, the environmental glucose competes with the glucose conjugated on the nanoparticle to bind with the PBA causing the dissociation the polymer chains conjugated with glucose from the polymer chains conjugated with PBA, and subsquently the release of the loaded insulin. The PBA in the nanoparticles can be replaced with concanavalin A (ConA) which binds glucose with high specificity and affinity (b), or pH-sensitive polymers that become highly charged at low pH caused by gluconic acid which is generated by enzymatic degradation of glucose by glucose oxidase enzyme (c). Graphs were adapted, with permission, from [112,115].