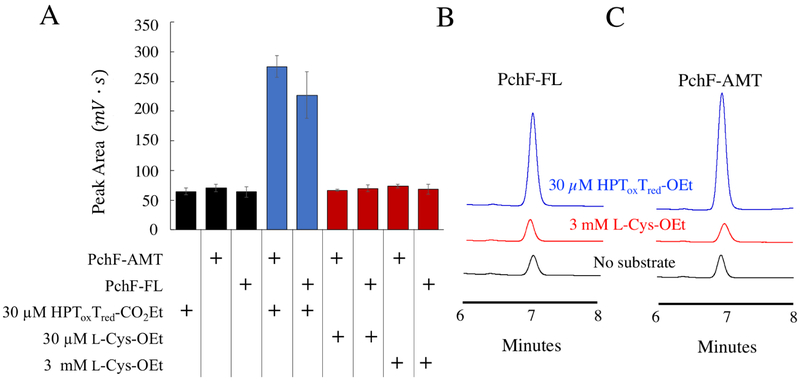
Figure 4: S-adenosylhomocysteine Formation Assay.
An orthogonal assay was used to determine if l-Cys-OEt was a substrate analog of PchF methyl transfer by detecting the formation of coproduct, AdoHCys, by UPLC separation. PchF-FL or PchF-AMT was incubated with 1 mM AdoMet and either no substrate, 30 μM HPToxTred-CO2Et (blue), or 30 μM or 3 mM l-Cys-OEt for 3 hours before being separated by UPLC. A) A bar graph showing the peak area quantitation of AdoHCys for reactions with 1) 30 μM HPToxTred-CO2Et but without enzyme; 2) 1 μM PchF-AMT without substrate; 3) 1 μM PchF-FL without substrate; 4) 1 μM PchF-AMT with 30 μM HPToxTred-CO2Et; 5) 1 μM PchF-FL with 30μM HPToxTred-CO2Et; 6) 1 μM PchF-AMT with 30 μM l-Cys-OEt; 7) 1 μM PchF-FL with 30 μM l-Cys-OEt; 8) 1 μM PchF-AMT with 3 mM l-Cys-OEt; and 9) 1 μM PchF-FL with 3mM l-Cys-OEt. Comparative UPLC traces for reactions using B) PchF-FL and C) PchF-AMT are displayed when using no substrate (black), 3mM l-Cys-OEt (red), and 30 μM HPToxTred-CO2Et (blue). AdoHCys formation is within error of negative controls when l-Cys-OEt is used as a substrate. In contrast, equimolar amounts of AdoHCys are generated when HPToxTred-CO2Et is used as a substrate analog.