Abstract
Rheumatic fever is a rare yet serious condition develop as a consequence of throat infection caused by Streptococcus pyogenes. It is the leading cause for rheumatic heart disease. Rheumatic heart disease is a worldwide public health concern. It is a chronic condition that results in carditis, irreversible valve damage and heart failure in children and young adults living in low-income countries. The age of onset peaks between 5 and 15 years. Approximately, 3% of patients with untreated acute streptococcal sore throats develop rheumatic fever.
Rheumatic fever and rheumatic heart disease can be prevented with appropriate antibiotics administration to prevent the progression of valve damage. The current use of primary and secondary prevention antibiotics in Saudi Arabia is not known. Therefore, this clinical practice guideline is developed, based on the best available evidence, to promote appropriate antibiotics secondary prophylaxis use for prevention of rheumatic heart disease.
Keywords: RHD, RF, Penicillin, Antibiotic, Secondary prevention, Valvular disease, Valve replacement, Saudi, Guidelines
1. Introduction
Rheumatic heart disease (RHD) is one of the main causes of cardiovascular morbidity and mortality in young people leading to about 250,000 deaths per year worldwide [1]. Rheumatic fever (RF) is a rare and serious condition that has been known since 1812. In 1880 the association between sore throat infection causing RF and carditis was definitively linked. In 1960, RF was considered as one of the main leading reasons for death in children in the world [2], [3]. RHD is a worldwide public health concern. It is a chronic condition that results in valvular damage caused by multiple attacks by group A Streptococcus pyogenes. Although the occurrence of RHD has significantly decreased in developed countries it remains a major concern in developing regions such as Africa, south-central Asia and Arabian Gulf, including Saudi Arabia [4].
Rheumatic fever is a consequence of throat infection caused by Streptococcus pyogenes. This organism can cause a deleterious effect on susceptible untreated children [1]. It was previously shown by molecular mimicry that the antigens of Streptococcus pyogenes and human proteins could result in autoimmune reactions, both humoral and cell mediated, leading to RF and RHD [5]. It takes around 3 weeks post S. pyogenes infection to induce RF; causing an inflammation affecting brain, joint, skin andin irreversible valve damage and heart failure [6].
Generally, primary prevention of RF using the appropriate antibiotics to treat preceding Streptococcus pyogenes infection is considered the most effective method for preventing rheumatic heart disease. Moreover, RF can be prevented and controlled with regular antibiotics by inhibiting the risk for further S. pyogenes infections and causing progression of valve damage. Thus, heart valve surgery to repair or replace damaged heart valves can be prevented or delayed by using secondary prophylaxis antibiotics [7].
Considering the fact that Saudi Arabia is an endemic area for RHD, specific effort and guidelines are needed to streamline the practice.
This clinical practice guideline is based on the best available evidence, national and international, for the use of secondary prophylaxis antibiotics for the prevention of RHD. This guideline is developed with the consideration of the American College of Cardiology/American Heart Association (ACC/AHA) Task Force on Practice Guidelines, World Health Organization (WHO) Technical Report Series, Centers for Disease Control (CDC) and Prevention, and the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young.
2. Purpose of the guidelines
-
2.1
Because of the relatively high prevalence of RHD in our population and the increasing risk of rheumatic fever recurrence, adaptation of a national guideline on RHD to guide the use of antibiotics as prophylaxis in patients with rheumatic heart disease is paramount.
-
2.2
This guideline will enhance consistency in practice for the prevention of RHD.
-
2.3
This guideline may serve as a reference for healthcare professionals involved in the management of patients with RHD in their daily practice and to guide practitioners in selecting an appropriate regimen, dosing and duration of antibiotic therapy.
3. Epidemiology
Rheumatic Heart Disease is the leading cause of heart failure in children and young adults living in low-income countries. Globally, RHD is estimated to affect 15.6 million people resulting in 233,000 deaths annually. Re-hospitalization and heart surgeries as a result of RHD are highly significant from 5 years up to 20 years after diagnosis [8]. In the recent years, global burden of RHD have dramatically declined in developed country. On the other hand, RHD is still a major concern in many endemic countries. Approximately 1% of all schoolchildren show signs of RHD. Africa, Asia, Arab Gulf, the Pacific and indigenous populations of Australia and New Zealand are the areas worst affected by RHD [9], [10], [11]. Data on the prevalence of RHD among Saudi population is limited. However, percentage of children with RHD in Saudi Arabia remains above the global rate [12]. Moreover, The percentage of RHD patients presented with acute heart failure was reported to be 52%, while those who presented as high-risk chronic heart failure was 12%. These numbers are based on the HEARTS registry for acute and high-risk chronic heart failure [13]. In addition, two published studies reported a higher prevalence rates in children more than 5 years of age. In addition, two published studies reported a higher prevalence rates in children more than 5 years of age. According to the first study, out of 40 patients 34 had initial attacks and 12 recurrent cases [17]. The other study reported 51 initial attacks in children and 22 recurrences among 67 patients [15], (see Table 1). Rheumatic valvulitis leads to various degrees of valve involvement and destruction. Type of valve involved has an impact on the prevalence of rheumatic valvular lesions in Saudi Arabia (see Table 2) [14], [15], [16], [17].
Table 1.
| Author | Children | Follow up | RF | Initial | Recurrence |
|---|---|---|---|---|---|
| Al-Eissa YA et al. | 67 patients | 5 years | 73 episodes | 51 children 43% carditis |
22 children 91% carditis |
| Abbag F et al. | 40 patients | 9 years | 46 attacks | 34 attacks 67.6% carditis |
12 attacks 58.3% carditis |
RF = Rheumatic fever.
Table 2.
| Author | Children | MR | AR | MR and AR or TR |
|---|---|---|---|---|
| Al-Eissa YA et al. | 51 patients | 18 patients | 1 patient | 3 patients AR and MR |
| Abbag F et al. | 40 patients | 93.3% | 16.7% | 6.7% TR |
| Qurashi MA et al. | 83 patients | 58% | 9% | 25% AR and MR |
MR = Mitral Regurgitation, AR = Aortic Regurgitation, TR = Tricuspid Regurgitation.
4. Indication for antibiotics prophylaxis
All patients who have had rheumatic carditis, with or without valvular disease, are at high risk for RHD recurrence should receive long-term antibiotics therapy as secondary prevention. Prophylactic antibiotic therapy should be continued even after valve surgery, irrespective of the valve location or type (including mechanical and biological valves replacement), since these patients remain at risk for recurrence of RHD for the involved valve or other valves.
5. Antibiotic selection and duration of therapy
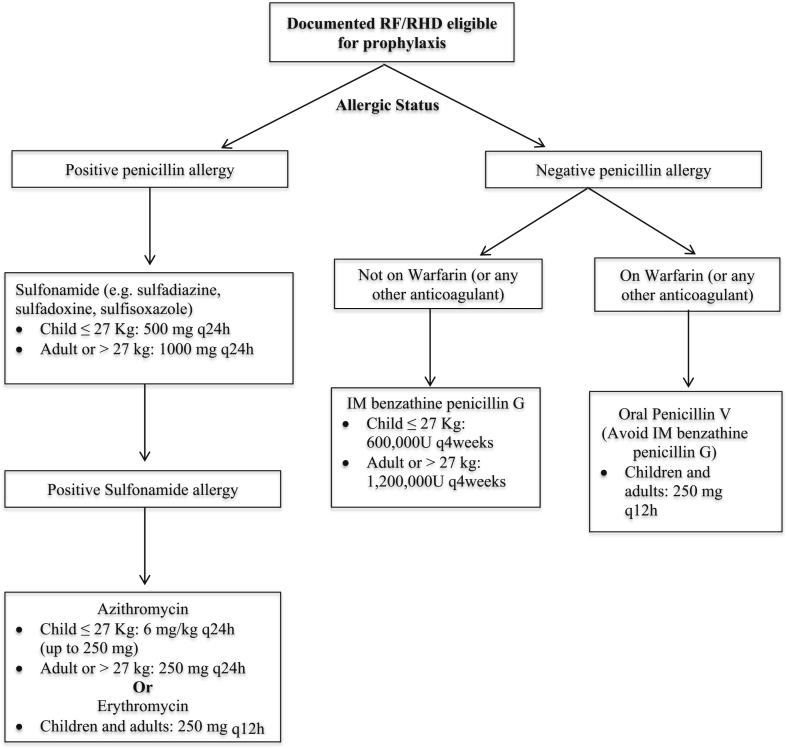
Secondary antibiotic prophylaxis is used to reduce the acquisition of new group A streptococcal strains that might induce repeated or chronic and acute rheumatic fever attacks, and is a major determinant of cardiac outcome. Medical intervention is based on the eradication of group A streptococcus with penicillin, which prevents the initial acute rheumatic fever attack (primary prophylaxis) or disease recurrences (secondary prophylaxis) [18]. Physicians select treatment and route of administration based on their assessment of patients' clinical condition and adherence to therapy(see Table 3, Figure). The duration of secondary prophylaxis depends on several factors including: patients' age, the date of their last attack, and most importantly the presence and severity of rheumatic heart (see Table 4) [18], [19], [20], [21].
Table 3.
Recommended antibiotics regimens for secondary prophylaxis of rheumatic fever and rheumatic heart disease [18], [19], [20], [21].
| Antibiotic | Child ≤27 Kg | Adult or > 27 kg | Route of administration |
|---|---|---|---|
| Agent of Choice | |||
| Benzathine benzylpenicillin Ga | 600,000 unitsb | 1,200,000 units | Single intramuscular injection every 4 weeks ce |
| Penicillin V | 250 mg q12 h | Oral | |
| For individuals allergic to Penicillin | |||
| Sulfonamide: “sulfadiazine” | 500 mg q24 h | 1000 mg q24 h | Oral |
| For individuals allergic to Sulfonamide or Penicillin | |||
| Erythromycine | 250 mg q12 hd | Oral | |
| Azithromycine | 6 mg/kg q24 h (up to 250 mg) | 250 mg q24 h | Oral |
Intramuscular injection should be avoided in all individuals receiving oral anticoagulant (i.e. warfarin).
For small children and infants Benzathine benzylpenicillin dose is 25,000 units per kg.
In high-risk population, administration every 3 weeks is justified and recommended in populations in which the incidence of rheumatic fever is particularly high and those who have recurrent acute rheumatic fever despite adherence to an every-4-week regimen.
Dosing for children: 20 mg/kg/day divided twice daily (maximum 500 mg per day; erythromycin is an acceptable alternative to azithromycin, although the latter has fewer adverse effects and permits once daily dosing).
Contraindications to macrolides: a. Hypersensitivity to macrolide antibiotics or any component of the formulation. b. History of cholestatic jaundice/hepatic dysfunction associated with prior azithromycin use. c. Altered cardiac conduction: Macrolides (especially erythromycin) have been associated with rare QTc prolongation and ventricular arrhythmias, consider avoiding use in patients with prolonged QT interval or concurrent use of Class IA (eg, quinidine, procainamide) or Class III (eg, amiodarone, dofetilide, sotalol) antiarrhythmic agents or other drugs known to prolong the QT interval.
Figure 1.
Algorithm for selection of the optimal secondary prophylaxis antibiotics in individual patients with RHD.
Table 4.
Duration of antibiotics as secondary prophylaxis for rheumatic fever and rheumatic heart disease [17], [18], [19], [20].
| Category of patient | Duration of prophylaxis |
|---|---|
| Rheumatic fever with carditis and residual heart disease (persistent valvular disease) | >10 years since last episode and at least until age 40 years, sometimes lifelong prophylaxisa |
| Rheumatic fever with carditis but no residual heart disease (no valvular disease) | For 10 years after the last attack, or at least until 21 years of age (whichever is longer) |
| Rheumatic fever without carditis | 5 years or until 21 years, whichever is longer |
| More severe valvular diseaseb | Lifelong |
| After valve surgery | Lifelong |
Patients who are at high risk and likely to come in contact with populations with high prevalence of streptococcal infection, i.e., teachers, day-care workers, clinical or Echocardiographic evidence.
Valve severity is diagnosed according to the following ECHO criteria: a. Valve area (cm2) < 1 in aortic, mitral and tricuspid valve. b. Mean gradient (mmHg): aortic >40, mitral >10, pulmonic >64, tricuspid >5.
6. Conclusion
These guidelines outline practical recommendations for secondary prevention of RHD. We also would like to stress on the fact that primary prevention of rheumatic fever is the optimal approach. We do believe that adapting national guideline will help in improving standards of care delivered to our patients, particularly for a chronic and progressive disease like RHD. However, adherence to the guideline will need a full awareness about the therapy among healthcare providers in our country.
A national level initiative for prevention and management of RHD should be top agenda in our healthcare system. Despite the fact that Saudi Arabia is geographically located in the regions of high prevalence of RHD, minimal data are available on the epidemiology of the disease and it's prognosis in our population. In spite, RHD remains a main cause for valve surgery. In the light of the scarcity of evidence, adherence to guideline is crucial.
It is a fact that limited structured evidence is available from North America and Europe basically because of the rarity of RF and RHD. Which increases the burden on clinicians in the region to generate evidence pertinent to our population and health care system.
Conflicts of interest
No conflicts of interest are reported.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
References
- 1.Gewitz M.H., Baltimore R.S., Tani L.Y., Sable C.A., Shulman S.T., Carapetis J. Revision of the Jones criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography a scientific statement from the American heart association. Circulation. 2015;131(20):1806–1818. doi: 10.1161/CIR.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 2.Fleming P.R. Recognition of rheumatic heart disease. Br Heart J. 1977;39:1045–1050. doi: 10.1136/hrt.39.10.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kadri S.M. Diagnosis of rheumatic fever. Indian J Pract Dr. 2005;2(1):3–4. [Google Scholar]
- 4.Seckeler M.D., Hoke T.R. The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease. Clin Epidemiol. 2011;3:67–84. doi: 10.2147/CLEP.S12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chopra P., Gulwani H. Pathology and pathogenesis of rheumatic heart disease. Indian J Patho Microbiol. 2007;50(4):685–697. [PubMed] [Google Scholar]
- 6.Marijon E., Mirabel M., Celermajer D.S., Jouven X. Rheumatic heart disease. Lancet. 2012;379:953–964. doi: 10.1016/S0140-6736(11)61171-9. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence J.G., Carapetis J.R., Griffiths K., Edwards K., Condon J.R. Acute rheumatic fever and rheumatic heart disease: incidence and progression in the northern territory of Australia, 1997 to 2010. Circulation. 2013;128:492. doi: 10.1161/CIRCULATIONAHA.113.001477. [DOI] [PubMed] [Google Scholar]
- 8.Carapetis J.R., McDonald M., Wilson N.J. Acute rheumatic fever. Lancet. 2005 Jul 9-15;366(9480):155–168. doi: 10.1016/S0140-6736(05)66874-2. [DOI] [PubMed] [Google Scholar]
- 9.Seckeler M.D., Hoke T.R. The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease. Clin Epidemiol. 2011;3:67–84. doi: 10.2147/CLEP.S12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendis S., Puska P., Norrving B. World Health Organization; Geneva: 2011. Global atlas on cardiovascular disease prevention and control. [Google Scholar]
- 11.Carapetis J.R. World Health Organization: WHO/FCH/CAH/05-07; Geneva: 2005. The current evidence for the burden of group a streptococcal diseases; pp. 1–60. [Google Scholar]
- 12.Carapetis J.R., Steer A.C., Mulholland E.K., Weber M. The global burden of group A streptococcus disease. Lancet Infect Dis. 2005;5(11):685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 13.AlHabib K.F., Elasfar A.A., AlBackr H., AlFaleh H., Hersi A., AlShaer F. Design and preliminary results of the heart function assessment registry trial. Eur J Heart Fail. 2011;13:1178–1184. doi: 10.1093/eurjhf/hfr111. [DOI] [PubMed] [Google Scholar]
- 14.Qurashi M.A. The pattern of acute rheumatic fever in children: experience at the children's hospital, Riyadh, Saudi Arabia. J Saudi Heart Assoc. 2009;21(4):215–220. doi: 10.1016/j.jsha.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Eissa Y.A., al-Zamil F.A., al Fadley F.A., al Herbish A.S., al-Mofada S.M., al-Omair A.O. Acute rheumatic fever in Saudi Arabia: mild pattern of initial attack. Pediatr Cardiol. 1993;14(2):89–92. doi: 10.1007/BF00796986. [DOI] [PubMed] [Google Scholar]
- 16.Al-Eissa Y.A. Acute rheumatic fever during childhood in Saudi Arabia. Ann Trop Paediatr. 1991;11(3):225–231. doi: 10.1080/02724936.1991.11747507. [DOI] [PubMed] [Google Scholar]
- 17.Abbag F., Benjamin B., Kardash M.M., al Barki A. Acute rheumatic fever in southern Saudi Arabia. East Afr Med J. 1998;75(5):279–281. [PubMed] [Google Scholar]
- 18.Nishimura R.A., Otto 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary. J Am Coll Cardiol. 2014;63(22):2438–2488. doi: 10.1016/j.jacc.2014.02.537. [DOI] [PubMed] [Google Scholar]
- 19.Wilson W., Taubert K.A., Gewitz M., Lockhart P.B., Baddour L.M., Levison M. Prevention of infective endocarditis: Guidelines from the American Heart Association. Circulation. 2007;116(15):1736–1754. doi: 10.1161/CIRCULATIONAHA.106.183095. [DOI] [PubMed] [Google Scholar]
- 20.Gerber M.A., Baltimore R.S., Eaton C.B., Gewitz M., Rowley A.H., Shulman S.T. Rheumatic fever and streptococcal pharyngitis. Circulation. 2009;119:1541–1551. doi: 10.1161/CIRCULATIONAHA.109.191959. [DOI] [PubMed] [Google Scholar]
- 21.Bisno A., Butchart E.G., Ganguly N.K., Ghebrehiwet T., Lue H.C., Kaplan E.L. Rheumatic fever and rheumatic heart disease: report of a WHO expert consultation. WHO Libr. 2001:923. [Google Scholar]