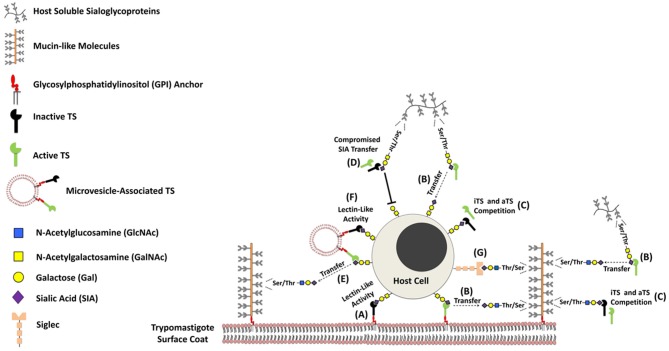
Figure 1.
Schematic model showing the presence of trans-sialidases and mucin-like molecules on the parasite cell surface. The biological properties of both GPI-anchored proteins (trans-sialidases [TS] and mucin-like molecules) have been extensively studied over the last years, and their immunobiological functions have been gradually disclosed. Trypanosoma cruzi expresses on its surface both inactive (iTS) and active (aTS) TS proteins, that present similar substrate specificity (α-2,3 SIA). While iTS displays lectinic-like activity (A), aTS shows the ability to modulate the sialoglycophenotype of both parasite and host cell glycans (B). Since both TS proteins compete by α-2,3 sialo-containing glycans (C), it may attenuate and or abrogate the process of SIA transfer mediated by aTS (D). Consequently, it might be able to compromise biological phenomena depend on the catalytic activity displayed by enzymatically active members. In addition, both TS may be found associated to microvisicles, displaying the same properties mediated by both fully soluble enzyme (E, F). The sialylation of glycoproteins found in the parasite cell surface besides to promote protection against soluble factors of the host immune system, may also provide ligand for SIA-binding proteins expressed by host cells, such as Siglecs (G). Since this phenomenon compromises the effective function of immune cells, it may represent an interesting mechanism to guarantee the perpetuation of the parasite in their infected host.