Table 1.
Optimization of the reaction conditions
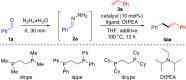
| ||||
|---|---|---|---|---|
| Entry | Catalyst | Ligand | Additive | 4aa (%)a,b |
| 1 | Ni(cod)2 | PMe3 | — | N.D. |
| 2 | Ni(cod)2 | PPh3 | — | N.D. |
| 3 | Ni(cod)2 | PCy3 | — | N.D. |
| 4 | Ni(cod)2 | dmpe | — | N.D. |
| 5 | Ni(cod)2 | dppe | — | N.D. |
| 6 | Ni(cod)2 | dcype | — | 78 (83:17) |
| 7 | NiCl2 | dcype | — | Trace |
| 8 | NiBr2 | dcype | — | Trace |
| 9 | NiBr2•glyme | dcype | — | Trace |
| 10 | Ni(acac)2 | dcype | — | 10 (78:22) |
| 11 | Ni(cod)2 | dcype | NaI | 91 (87)c (>95:5) |
| 12d | Ni(cod)2 | dcype | NaI | 85 (>95:5) |
| 13e | Ni(cod)2 | dcype | NaI | 71 (>95:5) |
| 14f | Ni(cod)2 | dcype | NaI | 58 (>95:5) |
| 15g | Ni(cod)2 | dcype | NaI | 78 (>95:5) |
| 16h | Ni(cod)2 | dcype | NaI | 69 (>95:5) |
| 17 | Ni(cod)2 | — | NaI | N.D. |
| 18 | — | dcype | NaI | N.D. |
Reaction conditions: 3a (0.2 mmol), 1a (0.6 mmol), N2H4•H2O (0.72 mmol), Ni(cod)2 (10 mol%), ligand (20 mol% for monodentate, 10 mol% for bidentate), DIPEA (0.4 mmol), NaI (0.1 mmol), THF (1.0 mL), 100 °C, 12 h under N2 unless otherwise noted
N.D. not detected
aNMR yields were determined by 1H NMR using mesitylene as an internal standard and based on 3a
bThe E:Z ratio in parenthesis was determined by 1H NMR analysis of the crude mixture
cThe isolated yield in parenthesis
d1a (0.4 mmol), N2H4•H2O (0.48 mmol) instead
e1a (0.3 mmol), N2H4•H2O (0.36 mmol) instead
f1a (0.2 mmol), N2H4•H2O (0.24 mmol) instead
gNi(cod)2 (5 mol%), ligand (5 mol%) instead
hWithout adding DIPEA
