Abstract
Objectives
Glucocorticoid (GC) treatment inhibits activation of runt-related transcription factor 2 (Runx2), which is essential for osteoblast differentiation from stem cells. As a result, GC treatment results in bone loss, GC-induced osteoporosis (GIO), elevated fracture risk, and delayed bone healing. Bisphosphonates such as alendronate (ALN) are recommended for treating or preventing GIO, and low-intensity pulsed ultrasound (LIPUS) facilitates fracture healing and maturation of regenerated bone. Combined therapy with ALN and LIPUS may stimulate cancellous bone healing in GIO rats. Here, we examined the effect of ALN and LIPUS on cancellous bone osteotomy repair in the proximal tibia of GIO rats.
Methods
Prednisolone (10 mg/kg body weight/day) was administered for 4 weeks to induce GIO in 6-month-old female Sprague-Dawley rats. Tibial osteotomy was then performed and daily subcutaneous injection of ALN (1-μg/kg body weight) was subsequently administered alone or in combination with LIPUS (20 min/day) for 2 or 4 weeks.
Results
ALN significantly increased bone mineral density (BMD) at 2 and 4 weeks, and ALN + LIPUS significantly increased BMD at 4 weeks. Bone union rates were significantly increased after 2 and 4 weeks ALN and ALN + LIPUS treatment. Lastly, ALN and ALN + LIPUS significantly increased the proportion of Runx2 positive cells at 4 weeks.
Conclusions
ALN monotherapy and combined ALN and LUPUS treatment augmented BMD and stimulated cancellous bone repair with increased Runx2 expression at the osteotomy site in GIO rats. However, the combined treatment had no additional effect on cancellous bone healing compared to ALN monotherapy.
Keywords: Glucocorticoid, Osteoporosis, Bisphosphonate
1. Introduction
Long-term administration of glucocorticoid (GC) results in significant bone loss, which can be as high as 8%–12% in the first several months of GC therapy and thereafter decreases by 2%–4% per year as long as GC therapy is continued [1]. GC therapy induces a form of osteoporosis known as glucocorticoid-induced osteoporosis (GIO), which is associated with bone loss as well as a higher fracture risk [2], [3], [4]. In addition to GIO, GC therapy causes a delay of fracture healing. Waters et al. [5] reported that the bone union rate and callus formation at the ulnar osteotomy site were delayed at 6 weeks after osteotomy in rabbits subjected to 2 months of prednisone treatment. Furthermore, several studies have reported that GC inhibits fracture healing in animal models [6], [7], [8]. Thus, it is very important to treat fractures in GIO patients completely and quickly, as these patients fracture easily and show impaired/delayed fracture healing. In addition to the treatment of fracture sites in GIO patients, treatment of general bone fragility in GIO patients is also very important to prevent subsequent insufficiency fractures.
To prevent or treat GIO, bisphosphonates are the first choice of the American College of Rheumatology recommendations [9] and the Japanese Society for Bone and Mineral Research [10]. Alendronate (ALN), which is one of the bisphosphonates, is commonly used for GIO prevention or treatment. Saag et al. [11] reported that ALN suppressed the decrease in bone mineral density (BMD) of the lumbar spine, proximal femur and trochanter in GIO patients. Furthermore, Adachi et al. [12] also reported that ALN decreased the incidence of vertebral fractures in GIO patients. However, concern could be raised that ALN treatment may impair healing process in GIO induced fragility fractures, because it exerts its suppressive effects on bone turnover.
Low-intensity pulsed ultrasound (LIPUS) is one of the methods used to accelerate fracture healing in fresh fractures and nonunions [13], [14], [15]. Our previous studies have demonstrated that LIPUS facilitates maturation of the regenerated cancellous bone at the osteotomy site of proximal tibia in aged rats [16] and combined treatment with ALN and LIPUS increased cancellous bone repair and bone strength in ovariectomized rats [17]. It has been considered that LIPUS is only useful method to accelerate a local fracture healing in delayed union of patients, who have a worse bone quality such as GIO.
However, to our knowledge, there are no studies investigating the combined effects of ALN and LIPUS on bone repair at the cancellous bone osteotomy of the proximal tibia in GIO rats. The present study aimed to investigate the effects of ALN and/or LIPUS on cancellous bone healing at the osteotomy site of the proximal tibia in GIO rats. We hypothesize that combined therapy with ALN and LIPUS produces a positive effect in stimulating cancellous bone healing in GC treated rats.
2. Methods
2.1. Animals
Six-month-old female Sprague-Dawley rats (Charles River Laboratory Inc., Kanagawa, Japan) were housed in a controlled environment at 22 °C with a 12-h light/dark cycle. The rats were allowed free access to water and pair-fed standard food (CE-2; Clea Japan Inc., Tokyo, Japan) containing 1.14% calcium, 1.06% phosphorus, and 250 IU vitamin D3 per 100 g.
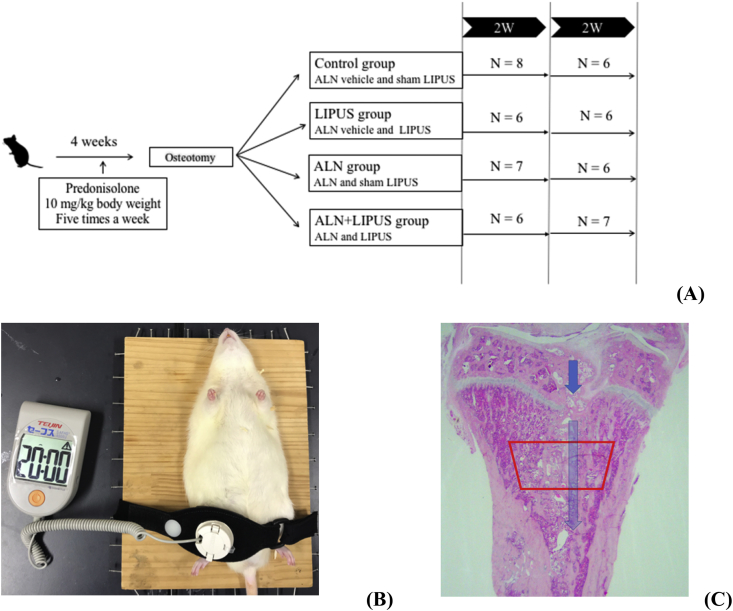
2.2. Experimental design (Fig. 1A)
Fig. 1.
Experimental protocol and LIPUS exposure. (A) Experimental protocol. (B) LIPUS treatment was administered to the right tibia of proximal tibia at the osteotomy site under the anesthesia. (C) Histological section stained with hematoxylin and eosin at the right proximal tibia including osteotomy site. The area framed by the red line, which is at 400 μm caudally from the lowest point of the growth plate and 100 μm medially from the endosteal surface, was region of interest for bone histomorphometry. ALN, alendronate, a daily subcutaneous injection of 1-μg/kg body weight; LIPUS, low-intensity pulsed ultrasound, a sonic Accelerated Fracture Healing System was exposed for 20 min per day; 2 W, 2 weeks.
We administered prednisolone to create the glucocorticoid-induced osteopenia (GIO) rats [18]. Prednisolone (prednisolone sodium succinate, Predonine; Shionogi, Osaka, Japan) was dissolved in saline and injected subcutaneously at a dose of 10-mg/kg body weight (BW)/day 5 times a week for 4 weeks. The rats (n = 66) were then randomized into 4 groups: (1) control group (saline administration with sham-LIPUS, n = 8 and 6 at 2 and 4 weeks, respectively), (2) LIPUS group (saline administration with LIPUS, n = 6 at 2 and 4 weeks), (3) ALN group (ALN administration with sham-LIPUS, n = 7 and 6 at 2 and 4 weeks, respectively), and (4) ALN + LIPUS group (ALN administration with LIPUS, n = 6 and 7 at 2 and 4 weeks, respectively). Fourteen rats were died during the experiment due to the anesthesia for LIPUS treatment. A cancellous bone osteotomy was performed on the right proximal tibia of each rat as previously described [19]. Briefly, a lateral parapatellar incision was made from the knee joint of the right hind limb through the proximal half of the tibia. An incomplete midsagittal osteotomy was performed from the joint surface around one-quarter of the proximal tibia, without extending to the caudal cortex using an electrical power saw (Yoshida Medical Inc., Tokyo, Japan). The site of the osteotomized tibia was closed using a nonabsorbable suture.
Postoperatively, there were no rats with an abnormal gait. ALN administration and/or LIPUS were started from the third day after the osteotomy and continued until the rats were sacrificed at 2 or 4 weeks. The right tibia from each rat was harvested and fixed in 10% neutral buffered formalin. All animal experiments were approved by the ‘‘Guidelines for Animal Experiments’’ of our institute (IACUC number: a-1-2609).
2.3. ALN administration
A solution of ALN (Wako Pure Chemical Co., Ltd, Osaka, Japan) was prepared in saline at a concentration of 0.02 mg/mL. Rats in the ALN and combination groups received a daily subcutaneous injection of ALN (1-μg/kg BW) for 7 days a week. This dose of ALN was equal to the dosage used in humans (5 mg/day) by oral administration, which is an approved dosage in Japan [20] and was chosen to be in accordance with that used in previous animal studies [16], [17]. Saline was selected as a vehicle control, and 0.2 mL of saline was injected subcutaneously in the control and LIPUS groups. BWs were measured weekly and the injection dosages were adjusted accordingly.
2.4. Ultrasound intervention (Fig. 1B)
LIPUS was provided by a Sonic Accelerated Fracture Healing System (SAFHS; Teijin Pharma, Tokyo, Japan). LIPUS signal strength and duration of treatment were consistent with the recommended clinical conditions for this device. The ultrasound signal generated with a transducer consisted of a burst width of 200 μs containing 1.5 MHz sine waves at a frequency of 1.0 kHz, and a spatial average-temporal average intensity of 30 mW/cm2. Rats were anesthetized with an intraperitoneal injection of ketamine (20-mg/kg BW) (Sankyo, Tokyo, Japan) and xylazine (1.5-mg/kg BW) (ZENOAQ, Fukushima, Japan) before exposure to LIPUS or sham-LIPUS 20 min per day for 7 days a week. Sufficient gel was used during the application of the ultrasound, and a rubber band was employed to fix the transducer against the antero-medial side of the osteotomized tibia such that the LIPUS could be routinely and consistently applied at the healing site.
2.5. Measurement of BMD
BMD of the entire excised tibia was measured by dual-energy X-ray absorptiometry (DXA, Hologic QDR-4500; Hologic, Marlborough, MA, USA) in the anterior plane. Bones were scanned in the ‘‘small animal’’ scan mode, with the ‘‘regional high-resolution’’ scan option. The region of interest (ROI) was 20 mm in length from the proximal edge of the tibia and the total width of the tibia [16], [17].
2.6. Sample preparation
After BMD measurements, the right proximal half of the tibia from each rat was decalcified with neutral 10% ethylene diaminetetraacetic acid for approximately 4 weeks and embedded in paraffin. Three micrometer-thick mid-frontal slices were then sectioned and stained with Hematoxylin and Eosin (H&E) for cancellous bone histomorphometry.
2.7. Bone histomorphometry
Bone histomorphometric analysis at the proximal tibia including osteotomy site with a magnification of × 200 was performed with a semiautomatic graphic system (Histometry RT CAMERA; System Supply, Nagano, Japan). Measurements were obtained at 400 μm caudally from the lowest point of the growth plate and 100 μm medially from the endosteal surface (Fig. 1C). The histomorphometric cancellous bone per tissue volume (BV/TV; %), osteoid surface (OS/BS; %), and eroded surface (ES/BS; %) were calculated as previously described [18].
2.8. Evaluation of bone union after osteotomy
H&E stained sections obtained from each osteotomized tibia were used to evaluate cancellous bone union. The semiautomatic graphic system, at × 100 magnification, was used to measure the length of bone union, defined as bone-to-bone bonding at the osteotomy line; these measurements were taken within the same area as that for the morphometry measurements. Cartilaginous bonding was also defined as bony union, whereas fibrous bonding was regarded as a nonunion. The proportion of bone union in the total length of the osteotomy line was calculated.
2.9. Immunochemistry
Other sets of sections of proximal tibiae, including the osteotomy site from each animal, were immunostained with a primary monoclonal antibody against runt-related transcription factor 2 (Runx2) (MBL Co., Ltd., Tokyo, Japan) to evaluate differentiation to osteoblasts. Four randomized ROI (size, 250 μm × 370 μm each) were analyzed at the osteotomy site in the right proximal tibia, which corresponded to the measurement area for bone histomorphometry [21].
2.10. Statistical analyses
All values are expressed as mean ± standard deviation. Two-factor factorial analysis of variance (ANOVA) was performed to evaluate the effect of ALN or LIPUS alone and the interaction between these interventions. Differences among groups at each time point were evaluated using Scheffe or Dunn post hoc tests for multiple comparisons using an ANOVA. All statistical analyses were performed using the Statistical Package for the Biosciences software (SPBS v 9.6; Akita University Graduate School of Medicine, Akita, Japan) [22]. Probability values of less than 0.05 were considered statistically significant.
3. Results
3.1. Bone mineral density
ALN treatment, but not LIPUS, significantly increased BMD at 2 and 4 weeks. ALN significantly increased the BMD at 2 and 4 weeks compared with that of the control. Combined treatment with ALN and LIPUS significantly increased the BMD at only 4 weeks compared with that of the control (Table 1).
Table 1.
Bone mineral density (BMD) at the osteotomy site of the proximal tibia.
| BMD (mg/cm2) | Control | LIPUS | ALN | ALN + LIPUS | Two-factor factorial ANOVA |
||
|---|---|---|---|---|---|---|---|
| LIPUS | ALN | Interaction | |||||
| 2 Weeks | 0.27 ± 0.02 | 0.27 ± 0.01 | 0.29 ± 0.02a | 0.28 ± 0.02 | NS | P = 0.009 | NS. |
| 4 Weeks | 0.25 ± 0.02 | 0.27 ± 0.03 | 0.31 ± 0.02b | 0.30 ± 0.02c | NS | P < 0.001 | NS. |
Values are presented as the mean ± standard deviation.
LIPUS, low-intensity pulsed ultrasound; ALN, alendronate.
Control, saline + LIPUS; LIPUS, saline + LIPUS; ALN, alendronate + sham LIPUS; ALN + LIPUS, alendronate + LIPUS; NS, not significant.
aP = 0.047, bP = 0.001, and cP = 0.002 vs. control group by 2-way analysis of variance (ANOVA) using Scheffe post hoc test.
3.2. Histological findings
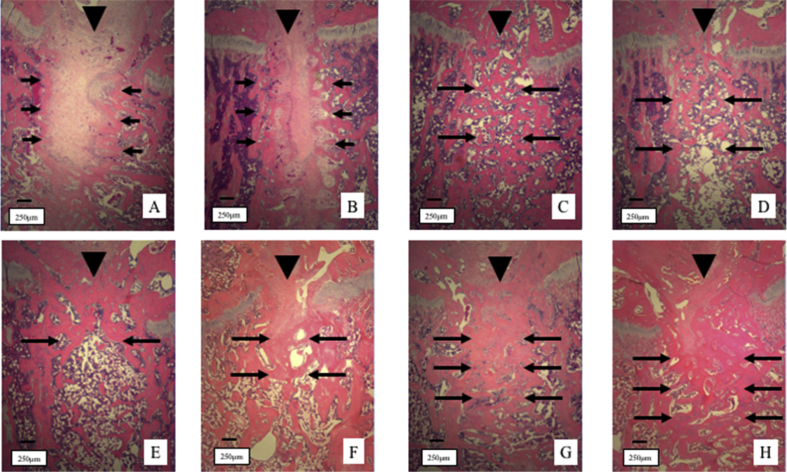
At 2 weeks of treatment, most of the areas at the osteotomy site were filled with fibrous tissues both in the control and LIPUS groups (Fig. 2A and B). On the other hand, cancellous bone to bone bonding was observed in the ALN and ALN + LIPUS groups (Fig. 2C and D). At 4 weeks of treatment, small amounts of fibrous bonding were found in the control and LIPUS groups (Fig. 2E and F). A thicker trabecular bone and dense trabecular bone union were observed in the ALN and ALN + LIPUS groups (Fig. 2G and H).
Fig. 2.
Histological sections of the osteotomy site stained with hematoxylin and eosin. Arrowhead (▼) indicates an interruption of the growth plate. At 2 weeks of treatment, most of the areas at the osteotomy site were filled with fibrous tissues (short arrows) in both the control (A) and LIPUS groups (B). On the other hand, bone to bone bonding (long arrows) was observed in the ALN (C) and ALN + LIPUS groups (D). At 4 weeks of treatment, small amounts of fibrous bonding were observed in the control (E) and LIPUS groups (F). A thicker trabecular bone and dense trabecular bone union were observed in the ALN (G) and ALN + LIPUS groups (H). LIPUS, low-intensity pulsed ultrasound; ALN, alendronate.
3.3. Bone histomorphometry at the osteotomy site of the proximal tibia
At 2 weeks after treatment, ALN contributed to the increases of BV/TV. However, the BV/TV showed no significant difference among the groups at 2 weeks. On the other hand, LIPUS increased the OS/BS. The OS/BS of the LIPUS and ALN + LIPUS groups were significantly higher than that of control group.
At 4 weeks of treatment, ALN significantly increased the OS/BS. The OS/BS in the ALN + LIPUS group was significantly higher than that of control group (Table 2).
Table 2.
Bone histomorphometric indices at the osteotomy site of the proximal tibia.
| Bone histomorphometry | Control | LIPUS | ALN | ALN + LIPUS | Two-factor factorial ANOVA |
||
|---|---|---|---|---|---|---|---|
| LIPUS | ALN | Interaction | |||||
| 2 Weeks | |||||||
| BV/TV (%) | 21.63 ± 7.78 | 19.39 ± 6.17 | 34.36 ± 12.32 | 31.75 ± 13.22 | NS | P = 0.004 | NS |
| OS/BS (%) | 2.59 ± 1.31 | 9.05 ± 3.87a | 7.05 ± 2.67 | 7.97 ± 3.82b | P = 0.032 | NS | NS |
| ES/BS (%) | 1.79 ± 1.29 | 3.76 ± 2.37 | 1.97 ± 1.33 | 2.56 ± 2.73 | NS | NS | NS |
| 4 Weeks | |||||||
| BV/TV (%) | 25.78 ± 8.04 | 23.08 ± 5.01 | 30.50 ± 8.28 | 28.26 ± 5.72 | NS | NS | NS |
| OS/BS (%) | 1.92 ± 1.70 | 4.21 ± 2.21 | 4.89 ± 1.72 | 5.86 ± 2.37c | NS | P = 0.011 | NS |
| ES/BS (%) | 3.06 ± 1.70 | 1.93 ± 1.42 | 2.03 ± 0.64 | 1.73 ± 1.19 | NS | NS | NS |
Values are presented as the mean ± standard deviation.
LIPUS, low-intensity pulsed ultrasound; ALN, alendronate.
Control, saline + sham LIPUS; LIPUS, saline + LIPUS; ALN, alendronate + sham LIPUS; ALN + LIPUS, alendronate + LIPUS; BV/TV, cancellous bone volume per tissue volume; OS/BS, osteoid surface per bone surface; ES/BS, eroded surface per bone surface; NS, not significant.
aP = 0.006, bP = 0.025, and cP = 0.021 vs. control group by 2-way analysis of variance (ANOVA) using Scheffe post hoc test.
3.4. Percentage of bony bonding at the osteotomy site of the proximal tibia
ALN treatment, but not LIPUS treatment, significantly increased the percentage of bone union at the osteotomy site at 2 and 4 weeks. The bone union rate of the ALN + LIPUS group was significantly higher than that of the control group at 2 weeks, but not of the ALN monotherapy group.
At 4 weeks of treatment, ALN significantly increased the percentage of bone bonding compared with the control. Furthermore, combined treatment with ALN and LIPUS significantly increased the percentage of bone union compared with the control as well as LIPUS mono-therapy. However, the combined therapy with ALN and LIPUS did not reveal significant increase in the cancellous bone repair compared with ALN monotherapy (Table 3).
Table 3.
Bone union rate at the osteotomy site of the proximal tibia.
| Bone union rate (%) | Control | LIPUS | ALN | ALN + LIPUS | Two-factor factorial ANOVA |
||
|---|---|---|---|---|---|---|---|
| LIPUS | ALN | Interaction | |||||
| 2 Weeks | 15.44 ± 13.58 | 17.05 ± 27.69 | 37.86 ± 19.50 | 46.82 ± 8.50a | NS | P = 0.001 | NS |
| 4 Weeks | 26.39 ± 6.18 | 30.44 ± 13.32 | 45.00 ± 14.24b | 49.35 ± 4.85c,d | NS | P < 0.001 | NS |
Values are presented as the mean ± standard deviation.
LIPUS, low-intensity pulsed ultrasound; ALN, alendronate.
Control, saline + sham LIPUS; LIPUS, saline + LIPUS; ALN, alendronate + sham LIPUS; ALN + LIPUS, alendronate + LIPUS; NS, not significant.
aP = 0.043, bP = 0.042, and cP = 0.007 vs. control group by 2-way analysis of variance (ANOVA) using Scheffe post hoc test. dP = 0.030 vs. LIPUS group by 2-way analysis of variance (ANOVA) using Scheffe post hoc test.
3.5. Immunohistochemistry of Runx2
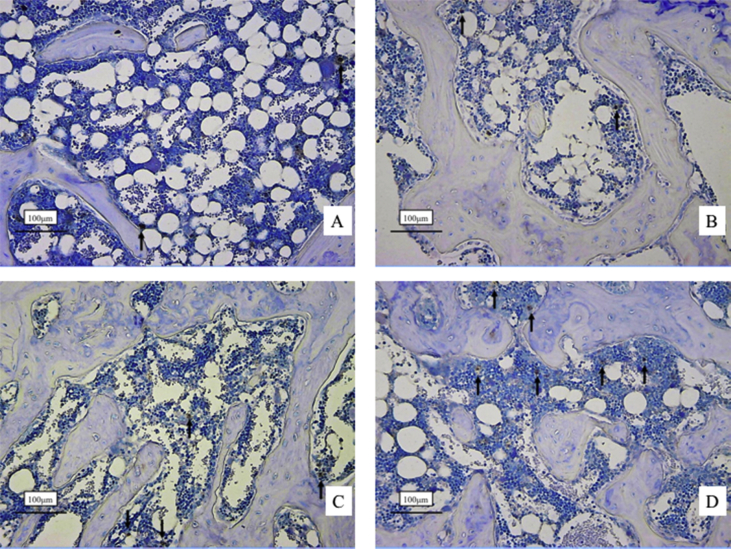
Histological sections of Runx2 immunostaining at 4 weeks are shown in Fig. 2. There are fewer Runx2 positive cells with dark reddish-brown dye in the control (Fig. 3A) and LIPUS group (Fig. 3B) than in the ALN (Fig. 3C) and ALN + LIPUS groups (Fig. 3D).
Fig. 3.
Immunostaining for Runx2 at the osteotomy site at 4 weeks. Immunostained positive cells stained dark reddish-brown dye. The long arrows indicate positive cells. There were fewer positive cells in both the control (A) and LIPUS groups (B) than the ALN (C) and ALN + LIPUS groups (D). Runx2, runt-related transcription factor 2. LIPUS, low-intensity pulsed ultrasound; ALN, alendronate.
Although only ALN increased the percentage of Runx2 positive cells at 2 and 4 weeks, there was no significance difference among the groups at 2 weeks. At 4 weeks of treatment, the percentages of Runx2 positive cells in the ALN and ALN + LIPUS groups were significantly higher than that of the control group (Table 4).
Table 4.
Percentage of Runx2-positive cells at the osteotomy site of the proximal tibia.
| Runx2-positive cell (%) | Control | LIPUS | ALN | ALN + LIPUS | Two-factor factorial ANOVA |
||
|---|---|---|---|---|---|---|---|
| LIPUS | ALN | Interaction | |||||
| 2 Weeks | 1.86 ± 1.69 | 1.89 ± 1.75 | 2.69 ± 1.16 | 4.20 ± 1.22 | NS | P = 0.011 | NS |
| 4 Weeks | 1.63 ± 0.43 | 1.89 ± 0.11 | 3.63 ± 0.22a | 3.46 ± 0.28b | NS | P < 0.001 | NS |
Values are presented as the mean ± standard deviation.
Runx2, runt-related transcription factor 2; LIPUS, low-intensity pulsed ultrasound; ALN, alendronate.
Control, saline + sham LIPUS; LIPUS, saline + LIPUS; ALN, alendronate + sham LIPUS; ALN + LIPUS, alendronate + LIPUS; NS, not significant.
aP < 0.001 and bP < 0.05 vs. control group by 2-way analysis of variance (ANOVA) using Dunn post hoc test.
4. Discussion
Here, we have demonstrated that the ALN mono-therapy and combined therapy with ALN and LIPUS increased the BMD and bone union rate of proximal tibial osteotomy site by increasing Runx2 expression in GIO rats. Whereas LIPUS mono therapy had no effect on neither BMD, bone union rate nor Runx2 expression. To our knowledge, this is the first report to investigate the effect of ALN and/or LIPUS in GIO rats including Runx2 expression.
In the present study, cancellous bone healing in GIO rats was 15.4% at 2 weeks. Our previous study reported that cancellous bone healing in aged rats was 40.0% at 2 weeks [16]. Thus, we suggest that cancellous bone healing in GIO rats was more delayed compared to the aged rats. In regard to the mechanisms responsible for impaired fracture healing under GIO conditions, several factors have been reported. GCs promote the transcriptional activity of peroxisome proliferator-activated receptor γ2, which promotes the differentiation of bone marrow mesenchymal cells to adipocytes and decrease the transcriptional activity of Runx2, which promotes the differentiation of mesenchymal stem cells to osteoblasts [23]. Furthermore, the Wnt signaling pathway, which is important for bone formation, is suppressed by GCs [24], [25]. GCs also inhibit the proliferation of osteoblasts and promote osteoblast apoptosis, resulting in markedly lower bone formation [26], [27].
In the clinical setting, ALN significantly increased lumbar-spine, hip, and total-body BMD in patients receiving GC therapy [12]. In this study, ALN intervention showed an increase in BMD compared to that of the control at 2 and 4 weeks. To treat GIO or delayed union of GC-induced osteoporotic fractures, ALN has been reported to inhibit Runx2 degradation, leading to suppressed bone resorption and apoptosis of osteoblasts [25]. Therefore, we have focused on the ALN effects on Runx2 expression at the osteotomy site in the GIO rats. ALN significantly increased the expression of Runx2 at the cancellous osteotomy site in GIO rats in the present study. Based on these results, we speculate that ALN stimulates the expression of Runx2 and suppresses apoptosis of osteoblasts, thereby resulting in increased BMD at the cancellous osteotomy site in GIO rats.
Numerous studies have been performed to clarify at a cellular level what occurs when LIPUS is applied to delayed or nonunion fracture sites. Chen et al. demonstrated that LIPUS treatment elevated Runx2 mRNA expression and progressively promoted osteocalcin mRNA expression in human osteoblasts [28], [29]. In the clinical setting, Suto et al. [30] reported a case of the effectiveness of LIPUS in the treatment of insufficiency fractures by long-term prednisolone administration. Therefore, we predicted that LIPUS mono-therapy would increase the expression of Runx2 and promote bone union. However, in this experiment, LIPUS mono-therapy did not increase the percentage of Runx2-positive cells and did not promote bone union at the osteotomy site of the proximal tibia in GIO rats.
We speculate that the negative effect of GC on osteoblasts is greater than the local positive effect of LIPUS. Our previous study reported that although LIPUS treatment did not stimulate cancellous bone union, combined treatment with ALN and LIPUS significantly increased the cancellous bone healing at the osteoporotic cancellous osteotomy site in the proximal tibia of ovariectomized rats compared with the LIPUS monotherapy [17]. Combined treatment with ALN and LIPUS also revealed significant increase in the cancellous bone repair compared with LIPUS monotherapy, but not ALN monotherapy, in GIO rats in the present study. These results indicated that LIPUS requires an additional treatment such as ALN for the restoration of decreased cancellous bone volume or impaired bone metabolism by GC therapy in order to exert its positive effects on fracture healing. In clinical situation, we consider that LIUPS is a useful treatment choice for delayed union of fractures in low bone quality patients such as GIO patients. However, an additional treatment with ALN may be necessary to exert LIPUS effects for fracture healing in GIO patients. Furthermore, ongoing treatment with a bisphosphonate, such as ALN, should be initiated immediately or as soon as possible in addition to surgery of the fracture site in patients who have been treated with GC and exhibit bone loss, and have fractures.
There are several limitations in this study. First, the osteotomy at the proximal tibia may be considerably different from the cancellous bone fractures in human patients encountered clinically, such as those in the vertebra, hip, forearm, or shoulder. At this time, however, animal models of cancellous bone fractures are limited compared with that of cortical bone repair. We have used this model as model of periarticular fractures where cancellous bone fractures are often occurred in the osteoporotic patients treated with GC. Second, this experiment was a model which ALN was started after proximal tibial osteotomy. This means that the treatment for osteoporosis was started after getting fractures in the osteoporotic patients. However, in clinical practice, there are several cases in which have already started ALN or other medicines for osteoporosis to prevent GIO before fracture occurrence. In that case, there may be possibility that the effects of ALN and LIPUS might be different. Therefore the extent experiment may be needed.
5. Conclusions
LIPUS mono-therapy did not show any significant effects on BMD or cancellous bone healing. On the other hand, ALN mono-therapy and combined treatment with ALN and LIPUS increased BMD and stimulated the cancellous bone repair with the expression of Runx2 at the osteotomy site in GIO rats. However, the combined treatment had no additional effect on cancellous bone healing compared to ALN monotherapy.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
The authors would like to thank Teijin Pharma, Tokyo, Japan, for kindly supplying the SAFHS system, and Ms. Matsuzawa for her support in performing the experiments.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Weinstein R.S. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62–70. doi: 10.1056/NEJMcp1012926. [DOI] [PubMed] [Google Scholar]
- 2.Van Staa T.P., Leufkens H.G., Abenhaim L., Zhang B., Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Min Res. 2000;15:993–1000. doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 3.van Staa T.P., Leufkens H.G., Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13:777–787. doi: 10.1007/s001980200108. [DOI] [PubMed] [Google Scholar]
- 4.Van Staa T.P., Laan R.F., Barton I.P., Cohen S., Reid D.M., Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum. 2003;48:3224–3229. doi: 10.1002/art.11283. [DOI] [PubMed] [Google Scholar]
- 5.Waters R.V., Gamradt S.C., Asnis P., Vickery B.H., Avnur Z., Hill E. Systemic corticosteroids inhibit bone healing in a rabbit ulnar osteotomy model. Acta Orthop Scand. 2000;71:316–321. doi: 10.1080/000164700317411951. [DOI] [PubMed] [Google Scholar]
- 6.Murakami H., Kowalewski K. Effects of cortisone and an anabolic androgen on the fractured humerus in Guinea pigs: clinical and histological study over a six-week period of fracture healing. Can J Surg. 1966;9:425–434. [PubMed] [Google Scholar]
- 7.Clein L.J., Kowalewski K. Some effects of cortisone and an anabolic steroid on healing of experimental fractures. Can J Surg. 1962;5:108–117. [PubMed] [Google Scholar]
- 8.Sissons H.A., Hadfield G.J. The influence of cortisone on the repair of experimental fractures in the rabbit. Br J Surg. 1951;39:172–178. doi: 10.1002/bjs.18003915411. [DOI] [PubMed] [Google Scholar]
- 9.Grossman J.M., Gordon R., Ranganath V.K., Deal C., Caplan L., Chen W. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res Hob. 2010;62:1515–1526. doi: 10.1002/acr.20295. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki Y., Nawata H., Soen S., Fujiwara S., Nakayama H., Tanaka I. Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research: 2014 update. J Bone Min Metab. 2014;32:337–350. doi: 10.1007/s00774-014-0586-6. [DOI] [PubMed] [Google Scholar]
- 11.Saag K.G., Emkey R., Schnitzer T.J., Brown J.P., Hawkins F., Goemaere S. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-induced osteoporosis intervention study group. N Engl J Med. 1998;339:292–299. doi: 10.1056/NEJM199807303390502. [DOI] [PubMed] [Google Scholar]
- 12.Adachi J.D., Saag K.G., Delmas P.D., Liberman U.A., Emkey R.D., Seeman E. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001;44:202–211. doi: 10.1002/1529-0131(200101)44:1<202::AID-ANR27>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 13.Gebauer D., Mayr E., Orthner E., Ryaby J.P. Low-intensity pulsed ultrasound: effects on nonunions. Ultrasound Med Biol. 2005;31:1391–1402. doi: 10.1016/j.ultrasmedbio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Heckman J.D., Ryaby J.P., McCabe J., Frey J.J., Kilcoyne R.F. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Jt Surg Am. 1994;76:26–34. doi: 10.2106/00004623-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Gebauer D., Correll J. Pulsed low-intensity ultrasound: a new salvage procedure for delayed unions and nonunions after leg lengthening in children. J Pediatr Orthop. 2005;25:750–754. doi: 10.1097/01.bpo.0000173245.12184.7e. [DOI] [PubMed] [Google Scholar]
- 16.Aonuma H., Miyakoshi N., Kasukawa Y., Kamo K., Sasaki H., Tsuchie H. Effects of combined therapy of alendronate and low-intensity pulsed ultrasound on metaphyseal bone repair after osteotomy in the proximal tibia of aged rats. J Bone Min Metab. 2014;32:232–239. doi: 10.1007/s00774-013-0492-3. [DOI] [PubMed] [Google Scholar]
- 17.Sato C., Miyakoshi N., Kasukawa Y., Tsuchie H., Kinoshita H., Ohuchi K. Effects of alendronate and low-intensity pulsed ultrasound therapies at osteoporotic cancellous osteotomy sites in proximal tibia of ovariectomized rats. J Orthop Res Ther. 2017 (in press) [Google Scholar]
- 18.Miyakoshi N., Sato K., Yoshida S., Abe T. Bone-loss pattern of corticosteroid-induced osteopenia in rats: a node-strut analysis of the tibia. J Bone Min Metab. 1997;15:94–99. [Google Scholar]
- 19.Nozaka K., Miyakoshi N., Kasukawa Y., Maekawa S., Noguchi H., Shimada Y. Intermittent administration of human parathyroid hormone enhances bone formation and union at the site of cancellous bone osteotomy in normal and ovariectomized rats. Bone. 2008;42:90–97. doi: 10.1016/j.bone.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 20.Shiraki M., Kushida K., Fukunaga M., Kishimoto H., Taga M., Nakamura T. A double-masked multicenter comparative study between alendronate and alfacalcidol in Japanese patients with osteoporosis. The alendronate phase III osteoporosis treatment research group. Osteoporos Int. 1999;10:183–192. doi: 10.1007/s001980050214. [DOI] [PubMed] [Google Scholar]
- 21.Tsuchie H., Miyakoshi N., Kasukawa Y., Aonuma H., Shimada Y. Intermittent administration of human parathyroid hormone before osteosynthesis stimulates cancellous bone union in ovariectomized rats. Tohoku J Exp Med. 2013;229:19–28. doi: 10.1620/tjem.229.19. [DOI] [PubMed] [Google Scholar]
- 22.Murata K., Yano E. Nankodo Publisher; Tokyo: 2002. Medical statistics for evidence-based medicine with SPBS user's guide. [Google Scholar]
- 23.Hofbauer L.C., Rauner M. Minireview: live and let die: molecular effects of glucocorticoids on bone cells. Mol Endocrinol. 2009;23:1525–1531. doi: 10.1210/me.2009-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohnaka K., Tanabe M., Kawate H., Nawata H., Takayanagi R. Glucocorticoid suppresses the canonical Wnt signal in cultured human osteoblasts. Biochem Biophys Res Commun. 2005;329:177–181. doi: 10.1016/j.bbrc.2005.01.117. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi K., Yamaguchi T., Yano S., Kanazawa I., Yamauchi M., Yamamoto M. BMP/Wnt antagonists are upregulated by dexamethasone in osteoblasts and reversed by alendronate and PTH: potential therapeutic targets for glucocorticoid-induced osteoporosis. Biochem Biophys Res Commun. 2009;379:261–266. doi: 10.1016/j.bbrc.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 26.Dempster D.W. Bone histomorphometry in glucocorticoid-induced osteoporosis. J Bone Min Res. 1989;4:137–141. doi: 10.1002/jbmr.5650040202. [DOI] [PubMed] [Google Scholar]
- 27.Weinstein R.S., Jilka R.L., Parfitt A.M., Manolagas S.C. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y.J., Wang C.J., Yang K.D., Chang P.R., Huang H.C., Huang Y.T. Pertussis toxin-sensitive Galphai protein and ERK-dependent pathways mediate ultrasound promotion of osteogenic transcription in human osteoblasts. FEBS Lett. 2003;554:154–158. doi: 10.1016/s0014-5793(03)01157-8. [DOI] [PubMed] [Google Scholar]
- 29.Takayama T., Suzuki N., Ikeda K., Shimada T., Suzuki A., Maeno M. Low-intensity pulsed ultrasound stimulates osteogenic differentiation in ROS 17/2.8 cells. Life Sci. 2007;80:965–971. doi: 10.1016/j.lfs.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 30.Suto K., Urabe K., Naruse K., Ueno M., Uchida K., Suto M. Low-intensity pulsed ultrasound to repair insufficiency fractures occurring adjacent to osteonecrosis caused by long-term steroid administration. Kitasato Med J. 2011;41:90–96. [Google Scholar]