ABSTRACT
Objectives
Our objective was to evaluate the in vitro activity of ceftolozane-tazobactam against multidrug resistant (MDR) and extensively drug-resistant (XDR) non metallo-β-lactamase producing Pseudomonas aeruginosa clinical isolates at Hospital Universitario Miguel Servet (Zaragoza, Spain) from February 2016 to October 2017.
Material and methods
We evaluated the in vitro activity of ceftolozane-tazobactam and other antipseudomonal antibiotics against 12 MDR and 117 XDR non metallo-β-lactamase producing P. aeruginosa isolates. Ceftolozane-tazobactam minimal inhibitory concentrations (MICs) were determined by MIC gradient diffusion test strip.
Results
Among the 129 MDR/XDR isolates included, 119 (92.2%) were susceptible to ceftolozane-tazobactam, and ten (7.8%) were resistant. MIC50 was 2 mg/L, and MIC90 4 mg/L. Ceftolozane-tazobactam was the second most active antibiotic after colistin, overtaking amikacin.
Conclusions
Ceftolozane-tazobactam is a valuable treatment option for MDR and XDR P. aeruginosa infections in our setting.
Keywords: Pseudomonas aeruginosa, multidrug resistance, extensively drug resistance, ceftolozane-tazobactam, beta-lactams
RESUMEN
Objetivos
Nuestro objetivo fue evaluar la sensibilidad in vitro de ceftolozano-tazobactam en aislados clínicos de P. aeruginosa multirresistente (MDR) y extremadamente resistente (XDR) desde Febrero de 2016 a Octubre de 2017 en el Hospital Universitario Miguel Servet, Zaragoza (España).
Material y métodos
Evaluamos la actividad in vitro de ceftolozano-tazobactam y otros antibióticos anti-pseudomónicos en 12 aislados de P. aeruginosa MDR y en 117 aislados XDR, no productores de metalo-β-lactamasas. Se determinó la concentración mínima inhibitoria (CMI) de ceftolozano-tazobactam mediante tiras de difusión en gradiente.
Resultados
Entre los 129 aislados MDR/XDR incluidos, 119 (92,2%) fueron sensibles a ceftolozano-tazobactam, y diez (7,8%) presentaron resistencia. La CMI50 fue de 2 mg/L, y la CMI90 de 4 mg/L. Ceftolozano-tazobactam fue el segundo antibiótico más activo después de colistina, superando a amikacina.
Conclusiones
Ceftolozano-tazobactam es una opción de tratamiento válida para infecciones causadas por P. aeruginosa MDR y XDR en nuestro entorno.
Palabras clave: Pseudomonas aeruginosa, multirresistente, extremadamente resistente, ceftolozano-tazobactam, beta-lactámicos
INTRODUCTION
Pseudomonas aeruginosa is a ubiquitous non-fermentative Gram-negative bacterium and one of the leading causes of nosocomial infections. A point-prevalence study lead by the European Centre for Disease Prevention and Control in 2011-2012 found that almost 9% of hospital-acquired infections were caused by P. aeruginosa, whereas a Spanish EPINE survey in 2016 identified it as the second cause of nosocomial infections (after Escherichia coli), being present in 10.5% of isolates [1]. P. aeruginosa is intrinsically resistant to many antibiotics due to the high inducible expression of multidrug efflux pumps, low levels of non-specific porins, necessary for penetration of hydrophilic antibiotics, and due to the inducible expression of β-lactamases, such as AmpC [2]. P. aeruginosa is also highly capable of developing acquired resistance through chromosomal mutations that can lead to overexpression of AmpC or efflux pumps or to downregulation or loss of OprD porin, one of the mechanisms responsible of imipenem and meropenem resistance. It can also acquire resistance by horizontal gene transfer: plasmid-borne expression of extended-spectrum β-lactamases (ESBL), aminoglycoside-modifying enzymes and 16S rRNA methylases have been described in P. aeruginosa [2].
The increasing spread of multidrug-resistant (MDR) and extensively-drug resistant (XDR) P. aeruginosa is a growing concern worldwide [3]. Recent epidemiological studies reported MDR prevalence in P. aeruginosa of around 15% and XDR-prevalence ranging between 2.6% and 9.6% from in- and outpatients in North America [4, 5]. In Spain, the prevalence of MDR strains is already above 30% [3, 6].
Ceftolozane-tazobactam is a combination of a novel cephalosporin with the β-lactamase inhibitor tazobactam, approved for the treatment of complicated intra-abdominal infections, complicated urinary tract infections, and currently under investigation for the treatment of ventilator-associated pneumonia [7]. Unlike the previous-generation cephalosporins, ceftolozane-tazobactam has demonstrated increased stability to AmpC β-lactamases; it is also unaffected by the loss of expression of OprD porin and by the overexpression of multidrug efflux pumps [7]. The activity of ceftolozane is compromised in the presence of carbapenemases, such as metallo-β-lactamases (MBL) [7]. Ceftolozane-tazobactam constitutes a valuable treatment option for MDR gram-negative pathogens and is currently the cephalosporin with the highest activity against P. aeruginosa [3, 8].
The objective of the present study was to evaluate the in vitro activity of ceftolozane-tazobactam against MDR and XDR non MBL producing P. aeruginosa clinical isolates, in order to assess its suitability as treatment in infections caused by MDR and XDR P. aeruginosa in our setting.
MATERIALS AND METHODS
Samples were obtained from in- and outpatients at Hospital Universitario Miguel Servet, Zaragoza (Spain), between February 2016 and October 2017. Bacterial identification was performed by MALDI-TOF mass spectrometry (Bruker Daltonics) and antimicrobial susceptibility testing by MicroScan WalkAway (Beckman Coulter). The following antibiotics were routinely tested: ceftazidime, cefepime, piperacillin-tazobactam, aztreonam, imipenem, meropenem, ciprofloxacin, levofloxacin, gentamicin, tobramycin, amikacin, and colistin. Current EUCAST breakpoints [9] were applied to define resistance/susceptibility.
Ceftolozane-tazobactam minimal inhibitory concentrations (MICs) were determined by MIC gradient diffusion test strip (Liofilchem). MDR isolates were defined as those non-susceptible to ≥ 1 agent in at least 3 antibiotic classes; XDR isolates were defined as those only susceptible to ≥ 1 agent in maximum 2 antibiotic classes [10]. MBL producing isolates were excluded from the analysis since ceftolozane-tazobactam is not active against them. MBL production was screened by phenotypic methods: combined disk test with imipenem 10 µg (Oxoid) and imipenem 10 µg with 750 µg of EDTA (Sigma-Aldrich) in-house added and by Neo-Rapid CARB Kit (Rosco); and confirmed by real-time PCR (Check Direct CPE Kit Check-Points Health B.V.).
RESULTS
During the period studied, a total of 1,421 P. aeruginosa non-duplicate isolates were obtained; of these, 141 (9.9%) were defined as MDR and 316 (22.2%) as XDR. A total of 68 (4.7%) isolates were MBL producing.
In a total of 129 MDR/XDR P. aeruginosa non-duplicate isolates (one isolate per patient) and non-MBL producing ceftolozane-tazobactam could be tested and were included in the study. Of the 129 isolates, 12 (9.3%) were defined as MDR and 117 (90.7%) as XDR. One hundred-and-eight (83.7%) patients were male, mean age (SD) was 63.9 (18.0) years; most were in-patients (76.8%). The majority of isolates were from respiratory samples (45.8%) and urine samples (39.5%). The rates of susceptibility to the antibiotics included in the panel and other characteristics are summarized in table 1. Most isolates were classified as XDR, with a median of two active antibiotics out of 12 tested (range: 0-8). All except one isolate were susceptible to colistin. Other antibiotics more frequently active against P. aeruginosa isolates were amikacin (78.2%) and tobramycin (41.9%). A few isolates were susceptible to imipenem (12.4%), meropenem (5.4%), ceftazidime (10.1%), cefepime (10.8%) and piperacillin-tazobactam (6.2%). Only 34 (26.3%) isolates were susceptible to all the β-lactams tested. Thirteen (10.1%) isolates were only susceptible to colistin, and 49 (38%) were only susceptible to colistin and amikacin.
Table 1.
Characteristics of the 129 MDR/XDR P. aeruginosa isolates.
| Characteristics | n (%) |
|---|---|
| Type of sample | |
| Respiratory | 59 (45.8) |
| Urine | 51 (39.5) |
| Surgical wound | 9 (7.0) |
| Blood | 2 (1.6%) |
| Catheter | 1 (0.8%) |
| Others | 7 (5.4) |
| Origin of patient | |
| In-patient | 99 (76.8%) |
| Out-patient | 30 (23.2) |
| MDR | 12 (9.3) |
| XDR | 117 (90.7) |
| Isolates susceptible to: | |
| Amikacin | 101 (78.2) |
| Gentamicin | 31 (24.0) |
| Tobramycin | 54 (41.9) |
| Ceftazidime | 13 (10.1) |
| Cefepime | 14 (10.8) |
| Piperacillin-tazobactam | 8 (6.2) |
| Ceftolozane-tazobactam | 119 (92.2) |
| Imipenem | 16 (12.4) |
| Meropenem | 7 (5.4) |
| Aztreonam | 0 (0) |
| Ciprofloxacin | 10 (7.0) |
| Levofloxacin | 5 (3.9) |
| Colistin | 128 (99.2) |
| Isolates only susceptible to colistin and amikacin | 49 (38) |
| Isolates only susceptible to colistin | 13 (10.1) |
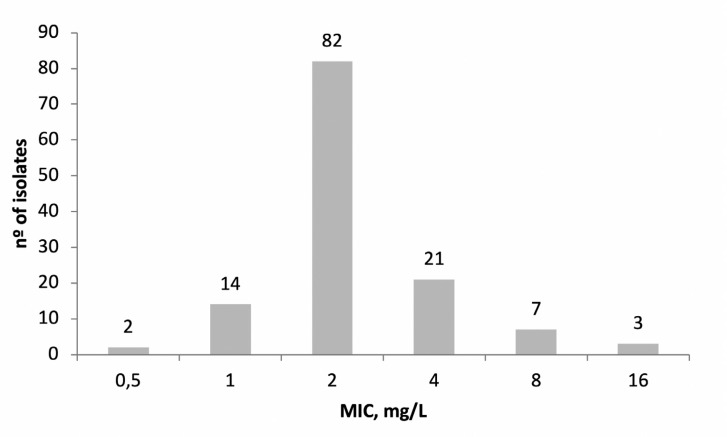
When susceptibility to ceftolozane-tazobactam was analyzed, 119 isolates (92.2%) were susceptible and 10 (7.8%) isolates showed MIC above the susceptibility breakpoint (4 mg/L); MIC50 was 2 mg/L, and MIC90 was 4 mg/L (figure 1). Among the 10 resistant isolates, five were susceptible to amikacin, and all were susceptible to colistin. Nine isolates were non-susceptible to the other β-lactams tested, and only one isolate was susceptible to imipenem and meropenem. Seven of the ten resistant isolates came from respiratory specimens.
Figure 1.
Distribution of ceftolozane-tazobactam minimal inhibitory concentrations (MIC) among the 129 P. aeruginosa isolates.
DISCUSSION
Although β-lactams are the mainstream therapy for P. aeruginosa infections, resistances to these drugs are increasingly common [1,3,4,5]. Thus, oprD porin mutation-mediated suppression causes resistance to carbapenems; overexpression of β-lactamase AmpC inactivates penicillins, cephalosporins and monobactams, whereas efflux pump up-regulation causes resistance to various β-lactams, fluoroquinolones and aminoglycosides. Plasmid-encoded ESBLs and MBLs are emergent and worrisome mechanisms of resistance in P. aeruginosa [1].
The combination of the fifth-generation cephalosporin ceftolozane, which is less affected by loss of OprD, overexpression of efflux pumps or AmpC than other available β-lactams, joined to tazobactam, which inhibits a wide variety of beta-lactamases (but not AmpC or MBLs), covers most of the pathogens causing β-lactam resistance [7]. Indeed, in the present study, only 7.8% of MRD/XDR non-MBL isolates were resistant to ceftolozane-tazobactam, whereas almost 75% of them were non-susceptible to the rest of the tested β-lactams. Two previous studies reported similar rates (from 4.8% to 7%) of non-MBL mediated resistance to ceftolozane-tazobactam in MDR and XDR P. aeruginosa [11,12].
In our study, we did not further explore the mechanisms of such resistance. When Moya et al. explored the mechanisms of pan-β-lactam resistance in P. aeruginosa (including ceftolozane), they found that all pan-resistant isolates lacked OprD, overexpressed AmpC and efflux pumps, and 5 out of 6 isolates had modifications in penicillin-binding protein (PBP) profiles [13]. An epidemiological study on a collection of 150 XDR isolates from Spanish hospitals reported an overall rate of non-susceptibility to ceftolozane-tazobactam of 31%, mediated by horizontally acquired carbapenamases, although potentially relevant PBP3 mutations were also detected [14]. Other studies identified AmpC overexpression and chromosomal mutations as potential mechanism of resistance to ceftolozane/tazobactam [15-17]. In our study, the ceftolozane-tazobactam MIC for non-MBL producing isolates ranged between 8 and 16 mg/L, which is only 2-4 fold higher that the breakpoint. It is reasonable to suggest that a massive overexpression of AmpC and/or the mutations, such as target modification, could lead to the observed non-MBL-mediated resistance.
Nonetheless, the activity of ceftolozane-tazobactam against the tested MDR and XDR isolates was high (92.2%) and was only surpassed by colistin. We conclude that ceftolozane-tazobactam is a valuable treatment option for complicated infections caused by MDR and XDR non-MBL producing P. aeruginosa isolates in our setting.
FUNDING
None to declare
CONFLICT OF INTEREST
Medical writing support was provided by Merck Sharp and Dohme (without being involved in manuscript content).
REFERENCES
- 1.Ruiz-Garbajosa P, Canton R. Epidemiology of antibiotic resistance in Pseudomonas aeruginosa. Implications for empiric and definitive therapy. Rev Esp Quimioter. 2017;30 Suppl 1:8-12. PMID: [PubMed] [Google Scholar]
- 2.Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front Cell Infect Microbiol. 2017;7:39. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mensa J, Barberán J, Soriano A, Llinares P, Marco F, Cantón R et al. . Antibiotic selection in the treatment of acute invasive infections by Pseudomonas aeruginosa: Guidelines by the Spanish Society of Chemotherapy. Rev Esp Quimioter 2018;31(1): 78-100 PMID: [PMC free article] [PubMed] [Google Scholar]
- 4.Sader HS, Huband MD, Castanheira M, Flamm RK. Pseudomonas aeruginosa Antimicrobial Susceptibility Results from Four Years (2012 to 2015) of the International Network for Optimal Resistance Monitoring Program in the United States. Antimicrob Agents Chemother. 2017;61(3). PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walkty A, Lagace-Wiens P, Adam H, Baxter M, Karlowsky J, Mulvey MR, et al. . Antimicrobial susceptibility of 2906 Pseudomonas aeruginosa clinical isolates obtained from patients in Canadian hospitals over a period of 8 years: Results of the Canadian Ward surveillance study (CANWARD), 2008-2015. J Diagn Microbiol Infect Dis. 2017;87(1):60-3. PMID: [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Barat L, Ferrer M, De Rosa F, Gabarrus A, Esperatti M, Terraneo S, et al. . Intensive care unit-acquired pneumonia due to Pseudomonas aeruginosa with and without multidrug resistance. J Infect. 2017;74(2):142-52. PMID: [DOI] [PubMed] [Google Scholar]
- 7.Zhanel GG, Chung P, Adam H, Zelenitsky S, Denisuik A, Schweizer F, et al. . Ceftolozane/tazobactam: a novel cephalosporin/beta-lactamase inhibitor combination with activity against multidrug-resistant gram-negative bacilli. Drugs. 2014;74(1):31-51. PMID: [DOI] [PubMed] [Google Scholar]
- 8.Sorbera M, Chung E, Ho CW, Marzella N. Ceftolozane/Tazobactam: a new option in the treatment of complicated gram-negative infections. P T. 2014;39(12):825-32. PMID: [PMC free article] [PubMed] [Google Scholar]
- 9.“The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 6.0, 2016 and Version 7.1, 2017 http://www.eucast.org.” [Google Scholar]
- 10.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. . Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268-81. PMID: [DOI] [PubMed] [Google Scholar]
- 11.Schaumburg F, Bletz S, Mellmann A, Becker K, Idelevich EA. Susceptibility of MDR Pseudomonas aeruginosa to ceftolozane/tazobactam and comparison of different susceptibility testing methods. J Antimicrob Chemother. 2017;72(11):3079-84. PMID: [DOI] [PubMed] [Google Scholar]
- 12.Wi YM, Greenwood-Quaintance KE, Schuetz AN, Ko KS, Peck KR, Song JH, et al. . Activity of Ceftolozane/Tazobactam Against Carbapenem-Resistant, Carbapenemase Non-Producing Pseudomonas aeruginosa and Associated Resistance Mechanisms. Antimicrob Agents Chemother. 2018; 62(1). PMC: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moya B, Beceiro A, Cabot G, Juan C, Zamorano L, Alberti S, et al. . Pan-beta-lactam resistance development in Pseudomonas aeruginosa clinical strains: molecular mechanisms, penicillin-binding protein profiles, and binding affinities. Antimicrob Agents Chemother. 2012;56(9):4771-8. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Barrio-Tofino E, Lopez-Causape C, Cabot G, Rivera A, Benito N, Segura C, et al. . Genomics and Susceptibility Profiles of Extensively Drug-Resistant Pseudomonas aeruginosa Isolates from Spain. Antimicrob Agents Chemother. 2017;61(11). PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haidar G, Philips NJ, Shields RK, Snyder D, Cheng S, Potoski BA, et al. . Ceftolozane-Tazobactam for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Infections: Clinical Effectiveness and Evolution of Resistance. Clin Infect Dis. 2017;65(1):110-20. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraile-Ribot PA, Cabot G, Mulet X, Periañez L, Martín-Pena ML, Juan C, et al. . Mechanisms leading to in vivo ceftolozane/tazobactam resistance development during the treatment of infections caused by MDR Pseudomonas aeruginosa. J Antimicrob Chemother. 2017. November 14. DOI: 10.1093/jac/dkx424 PMID: . [DOI] [PubMed] [Google Scholar]
- 17.Díaz-Cañestro M, Periañez L, Mulet X, Martin-Pena ML, Fraile-Ribot PA, Ayestarán I, et al. . Ceftolozane/tazobactam for the treatment of multidrug resistant Pseudomonas aeruginosa: experience from the Balearic Islands. Eur J Clin Microbiol Infect Dis. 2018. 37(11):2191-2200. DOI: 10.1007/s10096-018-3361-0 PMID: . [DOI] [PubMed] [Google Scholar]