Abstract
Background
Development of safe and effective chemopreventive agents is a winning strategy in reducing the morbidity and mortality of breast cancer. The current study was to investigate the mechanism-based chemopreventive potential of a Chinese herb formula Yanghe Huayan (YHHY) Decoction on the classical 7,12-dimethylbenz(a)anthracene (DMBA) induced rat mammary carcinogenesis model.
Methods
Female Sprague-Dawley rats at 42 days of age were orally administered with a human equivalent dose of YHHY Decoction at 0.02 ml/g (10 mg/ml) once daily, starting 1 wk. before and 4 wks following DMBA treatment. Mammary tumor occurrence was monitored every day. The length of time before palpable tumor is examined is defined as tumor-free survival time. High performance liquid chromatography (HPLC) analyses were adopted to identify major chemical compositions of the decoction. Following bioinformatics data mining and experimental analyses were performed to demonstrate the underlying mechanism of action.
Results
DMBA animals receiving YHHY Decoction exhibited a significant delay (P = 0.014) and in some animals prevention (P = 0.046) of tumor occurrence without obvious toxicity. Oncogenic myc activation was significantly suppressed in the DMBA-induced rats by the YHHY treatment. Eight major chemical compositions of the decoction were identified and were shown to interfere with multiple tumorigenic pathways simultaneously in the mammary tumors, including inducing tumor apoptosis and up-regulating pro-apoptotic protein Bax and down-regulating anti-apoptotic protein Bcl-2; suppressing abnormal cell proliferation and the MAPK/ERK, PI3K/AKT signalings; blocking neo-angiogenesis and the VEGF/KDR signaling, and inhibiting oxidative stress in the mammary tumors.
Conclusion
The multi-components and multi-targeting properties of the YHHY Decoction support its use as a potent chemopreventive drug in breast cancer.
Keywords: Chemoprevention, Mammary tumorigenesis, Multi-components, Multi-targeting, Chinese herb formula
Background
The prevalence of mammography screening, and the routine use of large core needle biopsies for the diagnosis of clinically occult breast lesions have led to an increased detection of early precursor lesions or precancerous breast conditions, such as atypical lobular hyperplasia (ALH), atypical ductal hyperplasia (ADH), lobular carcinoma in situ (LCIS) and ductal carcinoma in situ (DCIS) [1]. Several large cohort studies have agreed that the risk of breast cancer in women with ALH, ADH and LCIS is about 4–8 times higher than that of the general population [2–5]. ALH, ADH and LCIS are thought to be reversible, and the efficacy of chemoprevention with tamoxifen, raloxifene, and exemestane has been established in large prospective trials [6–9]. However, concerns about serious side effects preclude the widespread and long-term use of these medical options [10, 11]. On average, only 4% of high-risk women decide to take these chemopreventive drugs [12]. DCIS is referred as “stage zero breast cancer” and it has become one of the most commonly diagnosed breast conditions in recent years. Appropriate treatment for DCIS is crucial to prevent invasive breast cancer. However, although nearly all conceivable combinations of surgery, radiotherapy and systemic treatments have been tested in different trials, the situation is still unsatisfactory [13]. Hence, new breast cancer chemopreventive agents with acceptable efficacy and little toxicity that suitable for long-term use are in urgent need.
Traditional Chinese Medicine (TCM), with thousands of years clinical practice history, has been widely used for disease prevention and management, and is gaining popularity worldwide for promoting healthcare [14–16]. The advantages of “multi-component, multi-target and multi-pathway” combinational regulatory mechanism and relative low toxicity make the TCM herb formula promising for new drug development [17, 18]. We have used the Yanghe Huayan (YHHY) Decoction, which was originally derived from “Wai Ke Yi Jing” (Qing Dynasty) more than a century ago, to treat patients with chronic breast fibrosis and palpable lump in our clinic, and the average curative rate, defined as complete resolution or marked improvement of lumps and pain for at least two months with the herbal treatment, was 90% (560 women)(unpublished data). The YHHY Decoction is an aqueous preparation of herbal mixture and consists mainly of extracts from 8 Chinese medicinal herbs: Lu Jiao Jiao (Cornu Cervi Pantotrichum, CCP), Tu Beimu (Rhizoma bolbostemmae, RB), Bai Jie Zi (Semen brassicae, SB), Rou Gui (Cinnamomum cassia, CC), Pao Jiang (Baked ginger, BG), Ma Huang (Ephdra vulgaris, EV), Hu Tao Rou (Juglans regia L, JRL), and Sheng Gan Cao (Glycyrrhiza uralensis, GU). Although several individual herbs in the formula have been studied to have anti-tumor activity on cancer cell lines or tumor models [19–23], clinical benefits of these components on breast cancer prevention have ever been achieved only when mixing them together.
The current study investigated the underlying mechanism of the formula cocktail on preventing tumorigenesis in chemical carcinogen DMBA induced mammary tumors. Environmental chemical carcinogen exposure, majorly the polycyclic aromatic hydrocarbon chemicals (PAH), plays a significant role in causing breast cancer as evidenced by many epidemiological and laboratory studies [24, 25]. DMBA is a prototypical PAH that has been used extensively to induce mammary carcinomas in female Sprague-Dawley (SD) rats to study the process of carcinogenesis. Features of this process that make the model comparable to human disease include similarities in histologic progression, hormone dependence, angiogenic phenotype and oncogenic signaling activation [26–28]. In our study, YHHY Decoction was given to the animals starting 1 wk. before and 4 wks following DMBA induction as a chemopreventive regimen. Our results from both “dry” lab bioinformatics data mining and “wet” lab experimental analyses demonstrated that the YHHY Decoction exerted chemopreventive efficacy in the DMBA model of breast cancer by targeting multiple tumorigenic pathways simultaneously.
Methods
Preparation of YHHY decoction
The YHHY Decoction is an aqueous preparation from 8 Chinese medicinal herbs: CCP 15 g, RB 9 g, SB 6 g, CC 3 g, BG 3 g, EV 9 g, JRL 6 g, and GU 6 g. The raw herb materials were purchased from Bozhou Huqiao Pharmaceutical Co Ltd. (Anhui, China) in September 2015, and were authenticated by Dr. Feng Li at the Department of Pharmacognosy, Shandong University of TCM. A voucher specimen was deposited at TCM Pharmacy, Affiliated Hospital of Shandong University of TCM, with the voucher numbers as CCP 1508160116, RB 1507180227, SB 1507270896, CC 1508030119, BG 1506080417, EV 1507210325, JRL 1508200021, and GU 1507290632. Each raw herb was homogenized to fine powder and the eight herbs were mixed thoroughly. A 8.4 g sample of the mixed fine-ground powder was accurately weighted and extracted with 84 mL of boiling H2O for 6 h and then centrifuged at 12,000 rpm for 15 min, both the volatile oil and the supernatant were collected. The pellet was resolved in 60% ethanol (1:1.5 volume) and statically stewed for 24 h. The mixture was then centrifuged at 12,000 rpm for 15 min, and the supernatant was collected. The two supernatants were combined and dried in the rotary evaporator at 160 rpm at 30 °C. Then the dried powder was accurately weighed and dissolved in the volatile oil Tween-80 solution (1:1 volume) at 1 mg/ml.
Chemicals and reagents
DMBA was purchased from Sigma-Aldrich (St. Louis, MO). Primary antibodies, c-myc (NCL-cMYC, RRID:AB_563665, from Leica Microsystems), phosphor-c-myc (PA5–35814, RRID:AB_2553124, from Thermo Fisher Scientific), CD-105/Endoglin (NCL-CD105, RRID:AB_563482, from Leica Microsystems), and Ki-67 (sc-23,900, RRID:AB_627859), Bax (sc-70,407, RRID:AB_1119412), Bcl-2 (sc-7382, RRID:AB_626736), ERK1/2 (sc-135,900, RRID:AB_2141283), pERK1/2 (sc-136,521, RRID:AB_10856869), PI3K (sc-365,290, RRID:AB_10846944), AKT (sc-5298, RRID:AB_626658), KDR (sc-101,559, RRID:AB_1123212) and β-actin (sc-47,778, RRID:AB_2714189) from Santa Cruz Biotechnology (Santa Cruz, CA) were used. Antioxidant assay kit (cs0790) and In Situ Cell Death Detection Kit (11,684,817,910 ROCHE) were purchased from Sigma-Aldrich (St. Louis, MO). Ventana Basic DAB (3,3-diaminobenzidine) Detection kit was from Boster Biotechnology, Wuhan, China.
Animals
The animal study was conducted upon the approval by the Institutional Animal Care and Use Committee of Shandong University of TCM (SDUTCM2012042001). All experiments were performed in accordance with relevant guidelines and regulations. Pathogen-free virgin female Sprague-Dawley rats (MGI Cat# 5651135, RRID:MGI:5651135), approximately 42 days of age, weight 150 ± 10 g, were provided by Experimental Animal Center of Shandong University of TCM and housed in an animal facility accredited by the Chinese Association for the Accreditation of Laboratory Animal Care. The rats were acclimatized to standard housing conditions, including ambient temperature of 22 ± 2 °C, relative humidity at 30–50%, and a 12-h light-dark cycle, in plastic cages (maximum 4 animals/cage) for 1 wk. before initiation of the experiment. The animals had free access to the nutrition formula rodent diet (NTP-2000 standard) and drinking water.
Animal treatment protocol
The potential chemopreventive role of YHHY Decoction was investigated using a well-established DMBA-induced rat mammary tumorigenesis model [27, 29]. Following 1-wk acclimatization period, the rats were randomly divided into 4 groups with n = 6 per each group: group A-normal control with vehicle treatment; group B-DMBA induction with vehicle treatment, group C-DMBA induction with YHHY Decoction treatment and group D-normal control with YHHY Decoction treatment. The experiments were independently repeated three times, so the final sample size for each group is n = 18 rats. YHHY Decoction was fed through oral gavage (p.o.) once daily for 5 wks (1 wk. before and 4 wk. following DMBA treatment). A human equivalent dose of YHHY Decoction at 1 mg/ml, given by 0.02 ml/g body weight, was used based on the human to animal dose conversion formula (https://www.fda.gov/downloads/drugs/guidances/ucm078932.pdf, page 7). Following 1 wk. feeding with the YHHY Decoction, rats in the Groups B and C were administered a single dose of DMBA at 50 mg/kg body weight (dissolved in corn oil) by oral gavage to induce mammary carcinogenesis. This dose of DMBA was chosen so that substantial tumor incidence could be produced but not so high as to overwhelm chemopreventive action of YHHY Decoction. The specific time for DMBA exposure is based on previous carcinogenic bioassays indicating that rats at this age possess high frequency of terminal end buds that are sensitive to the established mammary carcinogen DMBA [30]. Rats in Group C were continually fed with YHHY Decoction for another 4 weeks after the DMBA administration (totally for 5 wks). Food, water intake and behavioral patterns were monitored daily, body weights were recorded twice a week. Palpation of mammary glands started 4 wks following DMBA treatment with a frequency of twice per week. The length of time before palpable tumor is examined is defined as tumor-free survival time. The experiments were terminated at 16 wks post-DMBA administration.
Tissue harvest and histopathology analyses
Animals were euthanized by cervical dislocation after overnight fast. The mammary tumors were carefully excised from mammary gland parenchyma and rinsed with phosphate-buffered saline (PBS) (pH = 7.4). Each tumor was measured in 2 perpendicular directions by a caliper to obtain an average diameter, then was cut into 2 halves. One half was immediately snap frozen in liquid nitrogen and used for molecular analysis. The other half was fixed in 4% paraformaldehyde and used for histopathological and immunohistochemical analysis. Serial tumor sections at 10 μm thickness were prepared for hematoxylin and eosin (H&E) and relevant immunohistochemistry staining. The mammary tumors were classified according the established criteria [31].
Immunohistochemical staining
Tumor sections were stained with primary Ki-67 antibody, and CD-105 antibody with the Ventana Basic DAB Detection kit for cell proliferation and microvessel detection. Apoptotic cells were detected by TUNEL reaction with In Situ Cell Death Detection Kit. The slides were evaluated by an independent pathologist who was blinded to group assignment and outcome assessment using a light microscope (BX41, Olympus). Five random fields under 20× objective for each slide, and at least 5 slides for each tumor sample were analyzed.
Tissue lysate antioxidant capacity analysis
Similar as previous described [32], two sets of measurements were performed on the excised mammary tissues: (i) antioxidant enzyme activities – superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT); and (ii) biochemicals – malondialdehyde (MDA) and total nitrate (NOx). Each frozen mammary tissue was divided into two portions (each approximately 0.5 g). One portion was homogenized in 1.5% ice-cold KCl solution to give a 10% suspension and used for the MDA assay. The other portion was cut into several small pieces, homogenized in PBS (pH 7.4), with a w/v ratio of 1:5, and spun at 13,000×g for 15 min, at 4 °C. The supernatant was separated for the measurements of SOD, CAT, GPx activities and NOx levels. The protein level in the supernatant was determined spectrophotometrically by the method of Lowry et al. [33] using BSA as a standard. SOD activity was expressed as the amount causing 50% inhibition of the reduction of cytochrome c per milligram of protein (U/mg of protein), with bovine copper–zinc SOD (Cu/Zn SOD) as standard [34]. CAT activity was measured by the method of Luck [35]. The decomposition of the substrate H2O2 was monitored spectrophotometrically at 240 nm. Specific activity was defined as micromole substrate decomposed per minute per milligram of protein (expressed as U/mg protein). GPx activity was measured as before [36]. Specific GPx activity (U/mg protein) was calculated as micromole NADPH consumed per minute per milligram of protein using an appropriate molar absorption coefficient (6220/M per cm). The level of MDA, determined using the method of Mihara and Uchiyama [37], was expressed as nmol/mg protein. The level of NOx was measured by the method developed by Sastry et al. [38]. A calibration standard involving potassium nitrate was used to calculate the total concentrations of nitrate, which was expressed as μmol/mg protein.
Western blot analysis
Equal quantities of protein from the tumor tissue lysate were processed for Western blotting. Each sample was denatured, electrophoresed, and transferred onto a PVDF membrane. After blocking the membrane, blots were incubated in specific primary antibodies and then secondary antibodies following the manufacturer’s instructions. Densitometric analysis was conducted using ImageJ software. The primary antibodies used include: anti-c-myc (1:5000), anti-phospho-c-myc (0.5 μg/ml), anti-Bax (1:1000), anti-Bcl-2 (1:2000), anti-ERK1/2 (1:500), anti-pERK1/2 (1:500), anti-PI3K (1:1000), anti-AKT (1:800), anti-KDR (1:400) and anti-β-actin (1:2000). No grouping of gels/blots cropped from different parts of the same gel or from different gels, fields, or exposures was performed.
HPLC analyses
The reference standards of eight chemical constituents, including adenosine, uracil, tubeimoside A, allyl isothiocyanate, trans-cinnamic acid, cinnamic aldehyde, 6-shogaol and polysaccharides (purity≥98%) were purchased from Chengdu Biopurify Phytochemicals Ltd., (Chengdu, China). Methanol, acetonitrile (Fisher Scientific, USA), and acetic acid (Tianjin Chemical Regent Co., Ltd., Tianjin, China) were of HPLC grade. The distilled water was obtained from Wahaha Co., Ltd. (Hangzhou, China).
An Agilent 1100 liquid chromatography system was used for the analysis, equipped with a diode array detector working in the range of 190-400 nm, a quaternary solvent delivery system, a column temperature controller, and an autosampler. The chromatographic data was recorded and processed with Agilent Chromatographic Work Station software.
For HPLC analysis and quantification of each constituents, 1 mg/ml decoction was filtered through a membrane filter (0.45 μm pore size) prior to injection and analyzed three times. Adenosine and uracil, cinnamic acid and cinnamaldehyde were examined simultaneously in one aliquot of the decoction sample, and other constituents were examined individually. Previously validated chromatographic conditions were applied to detect the concentrations of each constituent [39–45]:
For simultaneously detection of adenosine and uracil analysis, C18 analytical column (TIANHE Kromasil C18, 4.6 mm × 250 mm, 5 μm) was used. The mobile phase consisted of water (A)-acetonitrile (B); A:B was as follows: o min, 99:1; 5 min, 98:2; 10 min, 98:2; 20 min, 96:4; 42 min, 45:55; 55 min, 40:60; 60 min, 0:100. The flow rate and column temperature were set constantly at 0.3 ml/min and 30 °C. The detection wavelength was at 260 nm.
For simultaneously detection of cinnamic acid and cinnamaldehyde, C18 analytical column (Gemini C18, 4.6 mm × 250 mm, 5 μm) was used. The mobile phase consisted of solvent A (1.0%, v/v, aqueous acetic acid) and solvent B (1.0%, v/v, acetic acid in acetonitrile). The gradient flow of A:B was as follows: 0 min, 95:5; 40 min, 30:70; 45 min, 0:100, hold for 5 min; 55 min, 95:5, hold for 15 min. The flow rate and column temperature were set constantly at 1 ml/min and 40 °C. The detection wavelength was at 280 nm.
For detection of tuberimoside A, C18 analytical column (RP C18, 4.6 mm × 250 mm, 5 μm) was used. The mobile phase consisted of methanol-water (V:V = 68:32). The flow rate and column temperature were set constantly at 0.1 ml/min and 25 °C. The detection wavelength was at 214 nm.
For detection of allyl isothiocyanate, sphereclone ODS-2 column (4.6 mm × 15cmm, 5 μm) was used. The mobile phase was acetonitrile. The flow rate and column temperature were set constantly at 0.1 ml/min and 25 °C. The detection wavelength was at 242 nm.
For detection of 6-shogaol, C18 analytical column (Alltima HP C18, 4.6 mm × 250 mm, 5 μm) was used. The mobile phase consisted of water (A) and acetonitrile (B). The A:B flow was as follows; 0 min, 55:45; 8 min, 50:50; 17 min, 35:65; 32 min, 0:100 B, 38 min, 0:100, 43 min, 55:45, 48 min, 55:45. The flow rate and column temperature were set constantly at 1 ml/min and 30 °C. The detection wavelength was at 230 nm.
The reference standards were accurately weighed and dissolved in 60% ethanol, and then diluted to appropriate concentration ranges for the establishment of calibration curves. All stock and working standard solutions were stored in brown bottles at 4 °C until used for analysis.
Data mining using ingenuity pathway analysis (IPA)
Except tubeimosides A, other seven chemical constituents of the YHHY Decoction were found in the IPA database (Qiagen, USA), (RRID: SCR_008653) and their direct interacting molecules and endogenous chemicals, totally 293, were added to the analysis list. Core Analysis/Expression Analysis was performed using the Ingenuity Knowledge Base (RRID: SCR_008117) as a reference for p value calculation of the gene populations, and Experimentally Observed Direct Relationship was set to generate function networks.
Statistical analysis
Cumulative percentage of animals with tumor occurrence was plotted against time (weeks). Data were analyzed using Kaplan–Maier Analysis followed by Log-Rank test. The incidence of mammary tumor development in various groups was analyzed by Fisher’s exact probability test. Other data are presented as mean ± SD. Significant differences among various groups were determined by two-sided student t test followed by Holm-Sidak test. P < 0.05 was considered to be statistically significant. The commercial software SigmaPlot 11.0 (Systat Software, Inc., San Jose, CA) was used for all statistical analysis.
Results
General observations
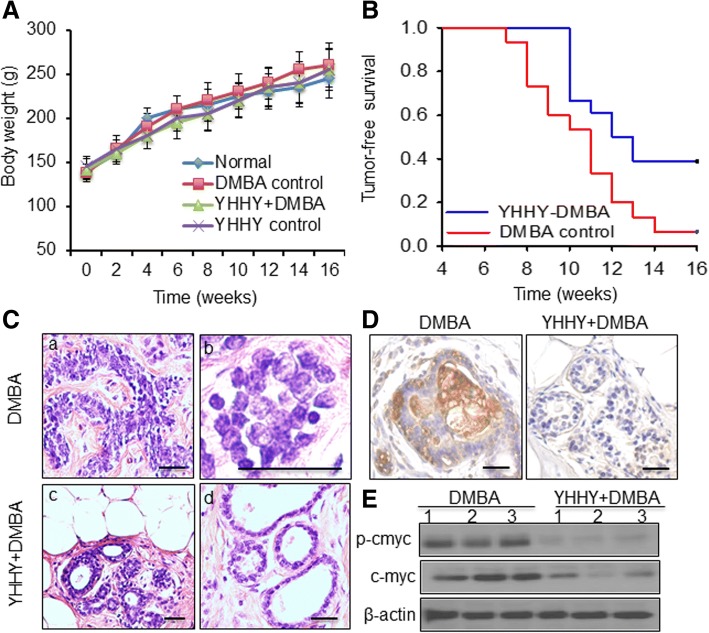
The well-established DMBA-induced rat mammary tumorigenesis model was used in the current study [27, 29]. There were 4 animal groups: group A-normal control with vehicle treatment; group B-DMBA induction with vehicle treatment, group C-DMBA induction with YHHY Decoction treatment and group D-normal control with YHHY Decoction treatment. The rats received YHHY Decoction gained their weight at similar rates as the group A rats (Fig. 1a). Food and water intake was monitored periodically throughout the study and did not differ among the treatment groups. There were no observed behavioral changes among animal groups (data not shown).
Fig. 1.
Effects of YHHY Decoction on DMBA-induced tumorigenesis and oncogenic myc activation. a. Effect of YHHY Decoction treatment on body weight gain during DMBA-induced mammary tumorigenesis in rats. Each data point indicates mean ± SD. There were 10–18 animals in various groups. No significant difference in body weights was observed among various rat groups at any time point. b. Effect of YHHY Decoction treatment on the occurrence of palpable tumors in the DMBA-induced rats. The length of time before palpable tumor is examined is defined as tumor-free survival time. n = 18 of the YHHY+DMBA group, and n = 14 of the DMBA control group. P = 0.014, Log rank test. c. Histopathology of the tumors detected in the DMBA control and YHHY Decoction treatment groups. DMBA control tumors showed extensive epithelial proliferation (a) and nuclear pleomorphism (b). Tumors treated with YHHY Decoction showed significantly improved cell architecture (c) and almost normal ductal and alveolar structure (d). Scale bar: 50 μm d. Immunohistochemical staining of phospho-c-myc in the mammary glands. Rats’ tissue were harvested two weeks after DMBA induction. The YHHY decoction treatment was initiated 1 week before DMBA induction and lasted for two weeks. Extensive staining of phospho-c-myc was observed in all examined animals in the DMBA group, while none of the animals in the YHHY prevention group showed obviously positive staining (n = 3). Scale bar: 50 μm e. Expressions of phospho-c-myc and total c-myc in 3 individual tumors in the YHHY Decoction treated vs. DMBA control group
YHHY decoction inhibited DMBA-induced tumorigenesis and oncogenic myc activation
The development of palpable tumors was first observed in the DMBA control rats 7 wks after DMBA induction (Fig. 1b). In contrast, rats fed with YHHY Decoction did not develop mammary tumors until wk. 10 after DMBA treatment (P = 0.015, log-rank test). At the end of the study which was 16 wk. post DMBA induction, while 94% (17/18) rats in the vehicle group were detected with palpable tumors, there was only 61% (11/18) rats receiving YHHY Decoction were found to bear tumors (P = 0.041, fisher exact test). Power analysis showed that having 18 animals in each group provided 100% power to detect the increase of the mean tumor-free survival time from 10.788 ± 0.646 wks in the DMBA control group to 12.944 ± 0.646 wks in the YHHY+DMBA group with alpha = 5%. These results indicated that YHHY Decoction treatment not only delayed the tumor latency, but also prevented the occurrence of mammary tumors in some rats. Tumors in the YHHY treatment group also showed 74% less of the average weight than those in the DMBA controls (5.1 ± 1.7 g vs. 19.5 ± 4.5 g, P < 0.05).
Histopathological examination on the tumor sections indicated that tumors in the group B (DMBA control) rats showed extensive epithelial proliferation and enlargement of the alveolus as a fused glandular pattern (Fig. 1c), as well as a presence of nuclear pleomorphism (Fig. 1c). Epithelial cells demonstrated various nuclear sizes and irregular chromatin (Fig. 1c). The YHHY Decoction treatment improved the cellular architecture remarkably. Most of the tissue appeared normal ductal and alveolar structure without hyperplastic changes on epithelial cells (Fig. 1c).
The terminal end buds in mammary gland comprise epithelial stem cells that are sensitive to carcinogen DMBA for malignant transformation [46]. Since myc proto-oncogene is a hallmark of cancer initiation [47], we first examined the expression of phosphorylated c-myc protein in the mammary glands upon DMBA induction and YHHY prevention. The results showed that two weeks after DMBA induction the mammary epithelial cells already exhibited elevated expression of phospho-c-myc, but its expression was absent in all the examined animals in the YHHY group (n = 3) (Fig. 1d). Besides tumor initiation, sustained myc activation also contributes to autonomous tumor proliferation and growth [47]. When examining the c-myc expression in all the endpoint tumors in the two groups (Fig. 1e), the results showed a significant decrease in the levels of phosphor-c-myc in tumors of YHHY-treated rats vs. tumors of the control rats (526 ± 83 vs. 831 ± 126, in relative densitometric units, P < 0.05). The concentrations of total c-myc protein were also decreased dramatically in the tumors of YHHY-treated rats (834 ± 118 vs. 1215 ± 185 in relative densitometric units, p < 0.05).
HPLC examination of eight chemical constituents in the YHHY decoction
HPLC analyses identified eight major chemical compositions of the decoction (6-shogaol, cinnamic acid, cinnamic aldehyde, polysaccharide, adenosine, uracil, tubeimoside A and allyl isothiocyanate) (Table. 1).
Table. 1.
HPLC detection of eight major chemical compositions of the YHHY decoction.
| Chemical constituents | Linear range (μg/ml) |
Limit of Quantity (μg/ml) | Concentration (μg/ml) |
|---|---|---|---|
| 6-shogaol | 4.5–30 | 1.5 | 22.5 ± 3.5 |
| cinnamic acid | 2.3–300 | 0.06 | 3.5 ± 0.05 |
| cinnamic aldehyde | 1.6–105 | 0.03 | 61.5 ± 5.6 |
| polysaccharide | 1.5–75 | 0.05 | 30.2 ± 4.6 |
| adenosine | 2.8–115 | 0.02 | 18.9 ± 2.6 |
| uracil | 2.7–230 | 0.01 | 11.1 ± 2.1 |
| tubeimoside A | 0.4–200 | 0.01 | 27.2 ± 2.3 |
| allyl isothiocyanate | 0.2–105 | 0.1 | 20.1 ± 3.7 |
Data are expressed as mean ± SD (n = 3)
Ingenuity pathway analysis (IPA) identified biological functions of the chemical constituents in YHHY decoction
The multifarious chemical constitutes of Chinese herbal cocktail may indicate complex mechanisms of action that warrant a systemic exploration. After searching in the IPA database, 7 out of the 8 chemicals were found and their direct-interacting molecules, totally 293 molecules, were used to analyze the disease or function networks related to the chemicals. Overall, the 293 molecules were significantly (P < 0.001) enriched in the top function networks of cell death and survival (apoptosis), cellular function and maintenance (cellular homeostasis), inflammatory response, organism injuries and abnormalities (inflammation of organ), free radical scavenging (synthesis of reactive oxygen species), cardiovascular system development and function (development of vasculature, angiogenesis), cell cycle (cell cycle progression), cellular movement (invasion of cells), and so on. Table. 2 listed the detailed category, disease and function annotation, p value and number of molecules for each network. Although needs further experimental validation on specific DMBA tumors, these results gave us an overall picture about the functions or mechanisms of the formula cocktail as a whole. In the following studies, we examined the activities of four major functions of the YHHY formula in the DMBA tumors.
Table. 2.
Top function networks of the YHHY Decoction chemical components and their 293 interacting molecules in IPA
| Categories | Disease or Function Annotation | p-Value | # Molecules |
|---|---|---|---|
| Cell Death and Survival | apoptosis | 5.16E-25 | 59 |
| Cellular Function and Maintenance | cellular homeostasis | 1.91E-22 | 46 |
| Tissue Morphology | Quantity of cells | 1.1E-22 | 42 |
| Cellular Development, Cellular Growth and Proliferation | Cell proliferation of tumor cell lines | 3.38E-21 | 41 |
| Inflammatory Response, Organism Injuries and Abnormalities | inflammation of organ | 5.02E-27 | 38 |
| Cardiovascular System Development and Function | development of vasculature, angiogenesis | 9.15E-22 | 37 |
| Free Radical Scavenging | synthesis of reactive oxygen species | 4.2E-24 | 36 |
| Cell Cycle | cell cycle progression | 5.47E-14 | 31 |
| Cellular Movement | invasion of cells | 1.08E-18 | 31 |
| Cancer, Cell Death and Survival, Tumor Morphology | Cell death of cancer cells | 1.28E-24 | 29 |
YHHY decoction induced tumor cell apoptosis, up-regulated Bax and down-regulated Bcl-2 expression
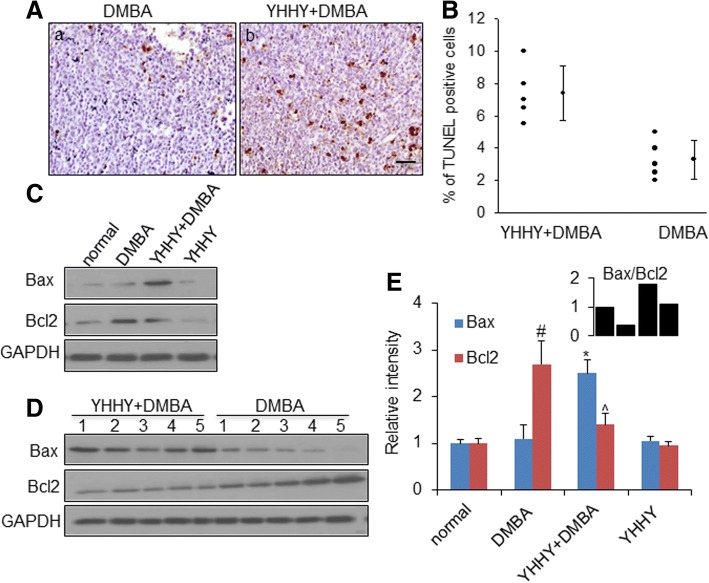
TUNEL assay was performed to evaluate the extent of cell apoptosis in the mammary tumor specimens. In the tumors from DMBA control rats, the presence of apoptotic cells was extremely rare. However, a significant increase of the apoptotic cell population was examined in the YHHY plus DMBA group (Fig. 2a, b).
Fig. 2.
YHHY Decoction induced tumor cell apoptosis. a. Representative TUNEL staining images showing the apoptotic cells in tumor sections in a: DMBA-induced rat and b: YHHY treated DMBA rat. Apoptotic cells were detected by TUNEL reaction with In Situ Cell Death Detection Kit. TUNEL-positive cells contain dark-brown nuclei. Scale bar: 50 μm. b. Quantitative analysis of the % of TUNEL positive cells in the tumor sections. P = 0.0023, student t test. n = 5 animals per group. c. Representative Western blot images of Bax and Bcl2 in one tumor sample from each group. d. Expressions of Bax and Bcl2 in 5 individual tumors in the YHHY Decoction treated vs. DMBA control group. e. Quantification of the western blot analysis for Bax and Bcl2 in normal mammary glands (normal and YHHY groups) and mammary tumors (DMBA and YHHY+DMBA groups). # p < 0.05 vs. normal; * p < 0.05 vs. DMBA; ^ p < 0.05 vs. DMBA. The up-right corner graph showing the ratio of Bax/Bcl2 in each group
To investigate the molecular signalings involved in the apoptosis-inducing effect of YHHY Decoction, Bax and Bcl2, two apoptosis-related proteins, both were in the 293 molecule list, were evaluated in the mammary tumors by Western blotting analysis. The Bax immuno-reactive band was detected extremely low in the DMBA control tumors (Fig. 2c, d). A significant increase of Bax expression was found in the tumors obtained from YHHY treatment group (Fig. 2c, d). The mammary tumors from DMBA control group showed extensive Bcl2 protein expression (Fig. 2c, d), and YHHY treatment exhibited considerable inhibition of Bcl2 expression (Fig. 2c, d). Oral administration of YHHY Decoction elevated the Bax/Bcl2 ratio under DMBA exposure (Fig. 2e). These data clearly indicated a pro-apoptotic mechanism of YHHY-mediated prevention of rat mammary tumorigenesis.
YHHY decoction inhibited abnormal tumor cell proliferation and the MAPK/ERK, PI3K/AKT signalings
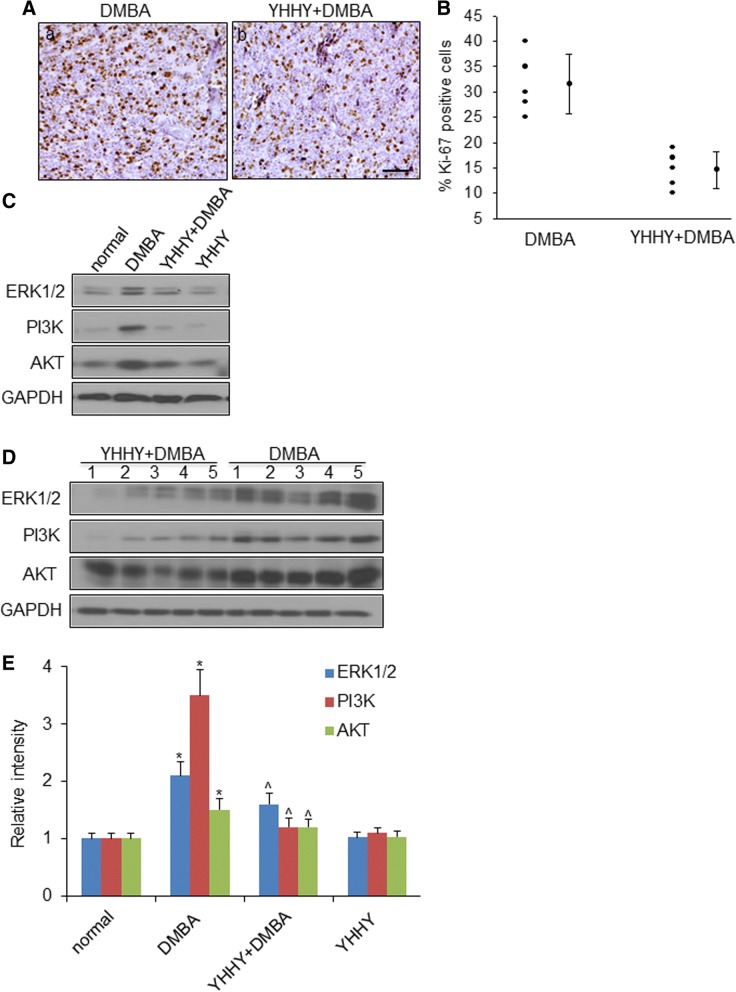
To determine whether YHHY Decoction impacted cell proliferation in the DMBA-induced mammary tumors, immunohistochemical staining of Ki-67 was assessed in the tumor sections. An abundant Ki-67-positive cells were found in the tumors from DMBA control rats, indicating active cell proliferation. YHHY Decoction suppressed cell proliferation to 30% of the DMBA tumors (Fig. 3a, b).
Fig. 3.
YHHY Decoction inhibited tumor cell proliferation. a. Representative images of Ki-67 immunohistochemistry staining showing the proliferative cells in tumor sections in a: DMBA-induced rat and b: YHHY treated DMBA rat. Tumor sections were stained with primary Ki-67 antibody and the DAB Detection kit for cell proliferation detection. Ki-67-positive cells contain dark-brown nuclei. Scale bar: 50 μm. b. Quantitative analysis of the % of Ki-67 positive cells in the tumor sections. P < 0.05, student t test. n = 5 animals per group. c. Representative Western blot images of ERK1/2, PI3K and AKT in one tumor sample from each group. d. Expressions of ERK1/2, PI3K and AKT in 5 individual tumors in the YHHY Decoction treated vs. DMBA control group. e. Quantification of the western blot analysis for ERK1/2, PI3K and AKT in normal mammary glands (normal and YHHY groups) and mammary tumors (DMBA and YHHY+DMBA groups). * p < 0.05 vs. normal; ^ p < 0.05 vs. DMBA
The activation of MAPK/ERK and PI3K/AKT signalings has been reported to contribute to both tumor initiation and oncogenic phenotype of mammary tumors through the effects on cell proliferation [1, 48]. ERK and AKT were both identified as the interacting molecules among the 293 molecules of the chemicals. Expressions of total ERK1/2, PI3K and AKT were examined to be 2–3 folds up-regulated in the DMBA control tumors compared to the normal mammary glands, and YHHY Decoction significantly suppressed their expressions (Fig. 3c, d).
YHHY decoction blocked neoangiogenesis and inhibited the VEGF/KDR signaling
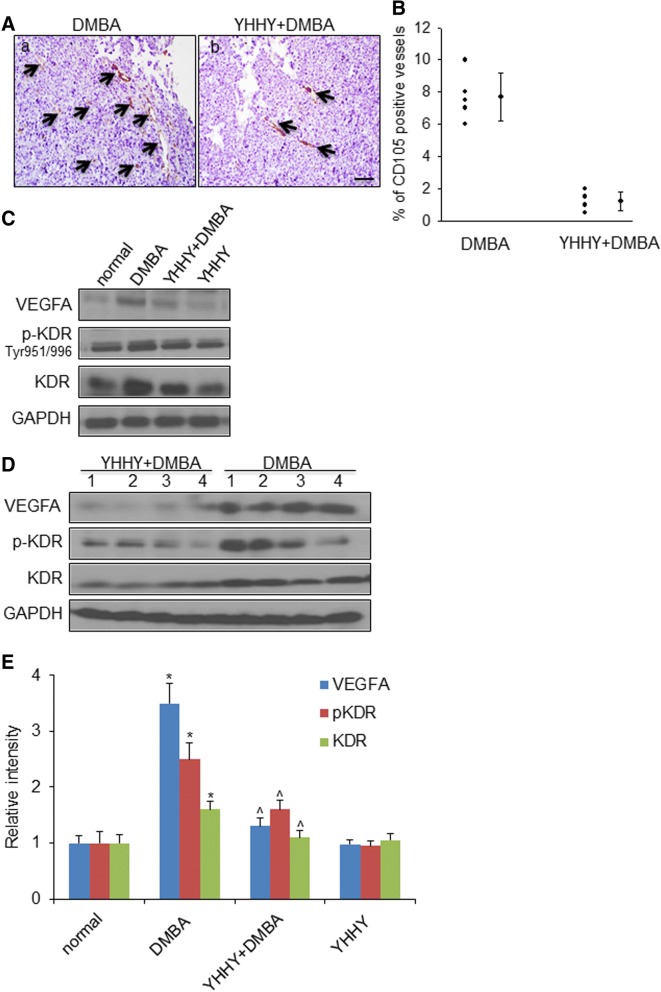
The onset of angiogenesis is believed to be an early event in tumorigenesis. Like human breast pathology, the progression of DMBA-initiated tumor in the rat is associated with an angiogenic phenotype [27] (Fig. 4a). CD-105/Endoglin antibody was used here to label only newly formed blood vessels [49]. A remarkable reduction (80%) of CD-105/Endoglin positive microvessel density was observed in the YHHY Decoction-treated DBMA tumors in comparing with the control DMBA tumors (Fig. 4a, b).
Fig. 4.
YHHY Decoction inhibited tumor neo-angiogenesis. a. Representative images of CD-105/Endoglin immunohistochemistry staining showing the newly formed blood vessels in tumor sections in a: DMBA-induced rat and b: YHHY treated DMBA rat. Black arrows point to the CD-105 positive blood vessels. Scale bar: 50 μm. b. Quantitative analysis of the vascular density in the tumor sections. P < 0.01, student t test. n = 5 animals per group. c. Representative Western blot images of VEGFA, p-KDR(Tyr 951/996) and total KDR in one tumor sample from each group. d. Expressions of VEGFA, p-KDR(Tyr 951/996) and total KDR in 4 individual tumors in the YHHY Decoction treated vs. DMBA control group. e. Quantification of the western blot analysis for VEGFA, p-KDR(Tyr 951/996) and total KDR in normal mammary glands (normal and YHHY groups) and mammary tumors (DMBA and YHHY+DMBA groups). * p < 0.05 vs. normal; ^ p < 0.05 vs. DMBA
Vascular endothelial growth factor (VEGF) and its receptor VEGFR2 or KDR play major roles in promoting the formation of new blood vessels [50, 51]. VEGFA and the receptor KDR were both examined to be expressed much lower in the YHHY treated tumors than those in the DMBA controls (Fig. 4c-e). While the less expressions of VEGF and its receptor may due to the less blood vessels in the YHHY treated DMBA tumors, we found that YHHY treatment blocked the activation of KDR at its major autophosphorylation site, i.e., Tyr951/996 [52] (Fig. 4c-e). These results suggested that the continuous use of YHHY Decoction in rats blocked the initiation and development of neoangiogenesis.
YHHY decoction inhibited oxidative stress in the mammary tumors
The IPA analysis also revealed that regulation of free radical scavenging is one of the possible mechanisms of action of the YHHY Decoction. Numerous studies have shown that free radicals reactive oxygen species are generated by exposure to DMBA [53, 54]. A balance between free radicals and antioxidants is necessary for proper physiological function. If free radicals overwhelm the body’s ability to regulate them, a condition known as oxidative stress ensues. Free radicals thus adversely alter lipids, proteins, and DNA and trigger a number of human diseases including cancer [55]. Thus, the antioxidant capacity of YHHY Decoction was measured by two sets of analyses: (1) anti-oxidant enzyme activities of SOD, GPx and CAT; and (2) biochemicals of MDA and total NOx. As shown in Table. 3, DMBA induction significantly stimulated the enzyme activity of SOD and GPx in the mammary tumors by 54 and 61%, respectively (P < 0.01). Treatment with YHHY Decoction completely blocked the enzymatic activation, reduced the mean value of the SOD enzyme activity similar to those of the control group (P = 0.41) and an even lower of the GPx activity (P = 0.02). DMBA induction decreased the CAT activity by 17% in comparing to the control group (P < 0.01), and the application of YHHY Decoction had no further effect (P = 0.44). In addition, DMBA significantly increased MDA and NOx levels by 36 and 54%, respectively (P < 0.01), but these increases were completely blocked by YHHY Decoction for MDA (P = 0.47) and NOx (P = 0.38). These results indicated a strong capacity of YHHY Decoction on anti-oxidative stress.
Table. 3.
Effects of YHHY Decoction treatment on antioxidant parameters in rats breast tissues
| Group | SOD (U/mg protein) |
GPx (U/mg protein) |
CAT (U/mg protein) |
MDA (nmol/mg protein) |
NOx (μmol/mg protein) |
|---|---|---|---|---|---|
| (A) Raw data | |||||
| A-Control (n = 10) | 2.68 ± 0.19 | 0.23 ± 0.02 | 624.5 ± 36.9 | 3.07 ± 0.37 | 5.84 ± 0.45 |
| B-DMBA (n = 14) | 4.12 ± 0.46 | 0.37 ± 0.06 | 520.1 ± 29.4 | 4.18 ± 0.42 | 8.99 ± 0.64 |
| C-YHHY+DMBA (n = 18) | 2.64 ± 0.33 | 0.19 ± 0.04 | 531.7 ± 31.7 | 3.02 ± 0.31 | 5.79 ± 0.47 |
| D-YHHY (n = 10) | 2.78 ± 0.12 | 0.24 ± 0.02 | 629.5 ± 29.2 | 3.19 ± 0.21 | 5.11 ± 0.39 |
| (B) Statistical comparison | |||||
| Group A vs B | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 |
| Group B vs C | < 0.01 | < 0.01 | 0.44 | < 0.01 | < 0.01 |
| Group A vs C | 0.41 | 0.32 | < 0.001 | 0.47 | 0.38 |
| Group A vs D | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 |
SOD superoxide dismutase, GPx glutathione peroxidase, CAT catalase, MDA malondialdehyde, NOx nitrate
(A) Raw data given as mean ± S.E.M. (B) Statistical analysis (Tukey’s two-way comparisons) of the data shown in part A
Discussion
The present study provides evidence for the first time that the ancient TCM herb cocktail YHHY Decoction exerts a notable chemopreventive activity in the experimentally-induced mammary tumorigenesis model, and more importantly, our bioinformatics and experimental approach systematically revealed the multi-components and multi-targeting mechanisms of the YHHY Decoction.
Our results indicate that daily oral intake of YHHY Decoction could significantly prevent or delay the development of mammary tumor in the rats. First, significantly lower percentage of YHHY-fed animals developed tumor within 16 wks after DMBA administration than the controls (61 vs. 94%, P = 0.041). Second, for the YHHY-fed rats that showed tumors, a much longer latent time was observed for the tumor development in compared to the controls (mean = 12.9 vs. 10.7 weeks, P = 0.015), and third, YHHY-treated tumors progressed much slowly than the control tumors within same time period, as manifested by a decreased tumor weight in the YHHY group (5.1 ± 1.7 vs. 19.5 ± 4.5, P < 0.05). These observations suggest that 1) the YHHY Decoction could substantially suppress the carcinogenic effect of DMBA; 2) the YHHY Decoction may selectively reverse the DMBA induced hyperplasia, thereby prohibit the development of breast cancer [13]. .
We observed suppressed myc activation by the YHHY decoction which may contribute to the prevention of tumorigenesis. Furthermore, the YHHY Decoction contains multiple bioactive components that work in a concert to modulate multiple dys-regulated pathways in precancerous cells and microenvironment (Table. 4). myc is one of the most potent oncogenes for cell transformation, but myc activation alone generally cannot induce tumorigenesis [47]. Tumorigenesis is a multifactorial process, in which carcinogenic factors disrupt the homeostatic molecular signaling networks, providing cells with essential physiological alterations to lead tumor formation. Sustained myc activation in a permissive epigenetic and/or genetic context orchestrates alterations of self-sustained growth, limitless replicative potential, angiogenesis, evasion of apoptosis, inflammation and oxidative stress [56–61]. These alterations are highly interconnected [56, 57]. For example, the carcinogen DMBA is demonstrated to induce high levels of oxidative stress in mammary gland [53, 54], subsequently induces overexpression of numbers of transcription factors (including c-myc) to promote the expression of genes related to cell proliferation, apoptosis and invasion [61]. To this end, the observed chemopreventive action of YHHY Decoction can be attributed to its capacity in regulating free radical scavenging and suppressing myc activation to potentiate tissue homeostasis.
Table. 4.
Composition and targeted pathways of the YHHY Decoction in preventing DMBA-induced mammary tumorigenesis
| Pinyin name | Botanical name | Examined chemical constituents | Targeted tumorigenic pathways |
|---|---|---|---|
| Lu Jiao Jiao | Cornu Cervi Pantotrichum | Adenosine Uracil |
protect tissues against excessive inflammation and promote tissue repair |
| Tu Beimu | Rhizoma Bolbostemmae | tubeimoside A | inhibit proliferation; induce apoptosis |
| Bai Jie Zi | Semen Brassicae | allyl isothiocyanate | induce apoptosis; inhibit proliferation; anti-angiogenesis |
| Rou Gui | Cinnamomum cassia | cinnamic acid cinnamic aldehyde |
antioxidant |
| Pao Jiang | Baked Ginger | 6-shogaol | induce apoptosis; anti-angiogenesis |
| Ma Huang | Ephdra Vulgaris | ||
| Hu Tao Rou | Juglans regia L | ||
| Sheng Gan Cao | Glycyrrhiza Uralensis | Polysaccharides | induce apoptosis; antioxidant |
Each component of the YHHY Decoction has been studied to regulate several different tumorigenic alterations, and several different components could regulate the same alteration pathways, which may implicate synergy [62, 63]. For example, 6-shogaol [64] has been shown to inhibit cancer cell growth and invasion, induce apoptosis and cancer cell differentiation, as well as suppress angiogenic factors [21, 65–67]. Cinnamic acid and cinnamic aldehyde have been identified with antioxidant, anti-inflammatory and cytotoxic properties [68]. Allyl isothiocyanate from Semen brassicae and tubeimoside A from Rhizoma bolbostemmae have been reported to inhibit tumor proliferation which was associated with cell cycle arrest and/or induction of apoptosis [23, 69, 70]. Allyl isothiocyanate also acts as an angiogenesis inhibitor in down-regulating VEGF and pro-inflammatory cytokines [71]. The polysaccharides from Glycyrrhiza uralensis exhibited antioxidant and immune-potentiation activities [40, 72]. Adenosine extracted from Cornu Cervi Pantotrichum can protect tissues against excessive inflammation and promote tissue repair [73]. Induction of apoptosis can be achieved by 6-shogaol, allyl isothiocyanate and tubeimoside A, same for antioxidant by cinnamic acid, cinnamic aldehyde and polysaccharides, and suppression of angiogenic factors by 6-shogaol and allyl isothiocyanate.
The IPA analysis helped us identify some key synergistic interactions occur on multiple tumorigenic signaling as many of the molecules interact with multiple components in the IPA database. For example, ERK1/2 is the interacting molecule with allyl isothiocyanate and adenosine, and Bcl2 is the interacting molecule with cinnamic aldehyde and allyl isothiocyanate. Although not all potentially bioactive constituents from the YHHY formula were identified, our study gained insight into the composition and mechanism by which YHHY Decoction abrogated mammary tumorigenesis in rats, and also demonstrated the holistic mode of action of the YHHY herb formula in targeting multiple systems.
Importantly, we didn’t observe any altered food intake, water intake, behavioral patterns, and growth rate of experimental animals upon YHHY treatment. This finding may indicate that the observed chemopreventive effect of YHHY Decoction is devoid of any toxic manifestation. Indeed, in comparing with synthesized chemical agents for anti-tumor growth, induction of apoptosis, anti-oxidant and anti-angiogenesis, the natural substances in the YHHY Decoction would be much less toxic [18]. Altogether, our study suggests that YHHY Decoction is a promising chemopreventive agent for breast cancer and merits further development.
Conclusions
In conclusion, our study demonstrated that the ancient TCM herb cocktail YHHY Decoction exerted a striking chemopreventive activity in the experimentally-induced mammary tumorigenesis model. Our bioinformatics and experimental approach systematically revealed the multi-components and multi-targeting mechanisms of the YHHY Decoction. These data supports the use of YHHY Decoction as a beneficial chemopreventive drug in breast cancer.
Acknowledgments
We thank Yuehua Jiang, Aili Song and Yian Sun for their excellent technical assistance and scientific discussion.
Funding
This study was supported by the National Science Foundation of China (No. 81573989 and 81403408 to JL, and No.81473687 to XL), and Shandong Science and Technology Program (No. 2016GSF202030) to JL.
Availability of data and materials
The data analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADH
Atypical ductal hyperplasia
- ALH
Atypical lobular hyperplasia
- BG
Baked ginger
- CAT
Catalase
- CC
Cinnamomum cassia
- CCP
Cornu Cervi Pantotrichum
- DCIS
Ductal carcinoma in situ
- DMBA
7,12-dimethylbenz(a) anthracene
- EV
Ephdra vulgaris
- GPx
Glutathione peroxidase
- GU
Glycyrrhiza uralensis
- H&E
Hematoxylin and eosin
- HPLC
High performance liquid chromatography
- JRL
Juglans regia L
- LCIS
Lobular carcinoma in situ
- MDA
Malondialdehyde
- NOx
Nitrate
- PAH
Polycyclic aromatic hydrocarbon chemicals
- PBS
Phosphate-buffered saline
- RB
Rhizoma bolbostemmae
- SB
Semen brassicae
- SD
Sprague-Dawley
- SOD
Superoxide dismutase
- TCM
Traditional Chinese Medicine
- VEGF
Vascular endothelial growth factor
- YHHY
Yanghe Huayan Decoction
Authors’ contributions
Conceived and designed the study: JL, XFL. Performed the study: JL, XFL, HC, ZS, HC, LW. Analyzed the data: JL, XL, HC, XS, XQL. Wrote the paper: JL. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal treatment and experiments were approved by the Institutional Animal Care and Use Committee of Shandong University of TCM (SDUTCM2012042001).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jingwei Li, Email: weilandetian2000@163.com.
Xiaofei Liu, Email: drliuxf@126.com.
Hongzhi Chen, Email: chz21century@126.com.
Ziyuan Sun, Email: ziyuanszy@163.com.
Hanhan Chen, Email: szychenhan@126.com.
Lei Wang, Email: raisehell@163.com.
Xiaohui Sun, Email: lancomegirl@163.com.
Xiangqi Li, Email: kemicui@gmail.com.
References
- 1.Sinn HP, Elsawaf Z, Helmchen B, Aulmann S. Early breast Cancer precursor lesions: lessons learned from molecular and clinical studies. Breast Care (Basel) 2010;5(4):218–226. doi: 10.1159/000319624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312(3):146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- 3.Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, Vierkant RA, Maloney SD, Pankratz VS, Hillman DW, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353(3):229–237. doi: 10.1056/NEJMoa044383. [DOI] [PubMed] [Google Scholar]
- 4.Degnim AC, Visscher DW, Berman HK, Frost MH, Sellers TA, Vierkant RA, Maloney SD, Pankratz VS, de Groen PC, Lingle WL, et al. Stratification of breast cancer risk in women with atypia: a Mayo cohort study. J Clin Oncol. 2007;25(19):2671–2677. doi: 10.1200/JCO.2006.09.0217. [DOI] [PubMed] [Google Scholar]
- 5.Warnberg F, Yuen J, Holmberg L. Risk of subsequent invasive breast cancer after breast carcinoma in situ. Lancet. 2000;355(9205):724–725. doi: 10.1016/S0140-6736(99)03703-4. [DOI] [PubMed] [Google Scholar]
- 6.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and bowel project P-1 study. J Natl Cancer Inst. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 7.Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, Norton L, Nickelsen T, Bjarnason NH, Morrow M, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281(23):2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 8.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon ER, Wade JL, 3rd, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila) 2010;3(6):696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, McTiernan A, Robbins J, Johnson KC, Martin LW, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364(25):2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 10.Zhou WB, Xue DQ, Liu XA, Ding Q, Wang S. The influence of family history and histological stratification on breast cancer risk in women with benign breast disease: a meta-analysis. J Cancer Res Clin Oncol. 2011;137(7):1053–1060. doi: 10.1007/s00432-011-0979-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coopey SB, Mazzola E, Buckley JM, Sharko J, Belli AK, Kim EM, Polubriaginof F, Parmigiani G, Garber JE, Smith BL, et al. The role of chemoprevention in modifying the risk of breast cancer in women with atypical breast lesions. Breast Cancer Res Treat. 2012;136(3):627–633. doi: 10.1007/s10549-012-2318-8. [DOI] [PubMed] [Google Scholar]
- 12.Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clinical Oncol. 2010;28(18):3090–3095. doi: 10.1200/JCO.2009.27.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lebeau A. Precancerous lesions of the breast. Breast Care (Basel) 2010;5(4):204–206. doi: 10.1159/000319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung F. TCM: made in China. Nature. 2011;480(7378):S82–S83. doi: 10.1038/480S82a. [DOI] [PubMed] [Google Scholar]
- 15.Normile D. DRUG DISCOVERY. Nobel for antimalarial drug highlights East-West divide. Science. 2015;350(6258):265. doi: 10.1126/science.350.6258.265. [DOI] [PubMed] [Google Scholar]
- 16.The Lancet O Rethinking traditional Chinese medicines for cancer. Lancet Oncol. 2015;16(15):1439. doi: 10.1016/S1470-2045(15)00406-4. [DOI] [PubMed] [Google Scholar]
- 17.Kong DX, Li XJ, Zhang HY. Where is the hope for drug discovery? Let history tell the future. Drug Discov Today. 2009;14(3–4):115–119. doi: 10.1016/j.drudis.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Ling CQ, Yue XQ, Ling C. Three advantages of using traditional Chinese medicine to prevent and treat tumor. J Integr Med. 2014;12(4):331–335. doi: 10.1016/S2095-4964(14)60038-8. [DOI] [PubMed] [Google Scholar]
- 19.Jo EH, Kim SH, Ra JC, Kim SR, Cho SD, Jung JW, Yang SR, Park JS, Hwang JW, Aruoma OI, et al. Chemopreventive properties of the ethanol extract of chinese licorice (Glycyrrhiza uralensis) root: induction of apoptosis and G1 cell cycle arrest in MCF-7 human breast cancer cells. Cancer Lett. 2005;230(2):239–247. doi: 10.1016/j.canlet.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 20.Gu Y, Korbel C, Scheuer C, Nenicu A, Menger MD, Laschke MW. Tubeimoside-1 suppresses tumor angiogenesis by stimulation of proteasomal VEGFR2 and Tie2 degradation in a non-small cell lung cancer xenograft model. Oncotarget. 2016;7(5):5258–5272. doi: 10.18632/oncotarget.6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray A, Vasudevan S, Sengupta S. 6-Shogaol inhibits breast Cancer cells and stem cell-like spheroids by modulation of notch signaling pathway and induction of Autophagic cell death. PLoS One. 2015;10(9):e0137614. doi: 10.1371/journal.pone.0137614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon HK, Hwang JS, So JS, Lee CG, Sahoo A, Ryu JH, Jeon WK, Ko BS, Im CR, Lee SH, et al. Cinnamon extract induces tumor cell death through inhibition of NFkappaB and AP1. BMC Cancer. 2010;10:392. doi: 10.1186/1471-2407-10-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y. Allyl isothiocyanate as a cancer chemopreventive phytochemical. Mol Nutr Food Res. 2010;54(1):127–135. doi: 10.1002/mnfr.200900323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller JA. Carcinogenesis by chemicals: an overview--G. H. A. Clowes memorial lecture. Cancer Res. 1970;30(3):559–576. [PubMed] [Google Scholar]
- 25.Pitot HC DY: Chemical carcinogenesis. In: Casarett and Doull’s Toxicology. edn. Edited by CD K. New York: McGraw-Hill; 1996: 201–267.
- 26.Russo J, Russo IH. Experimentally induced mammary tumors in rats. Breast Cancer Res Treat. 1996;39(1):7–20. doi: 10.1007/BF01806074. [DOI] [PubMed] [Google Scholar]
- 27.Heffelfinger SC, Gear RB, Taylor K, Miller MA, Schneider J, LaDow K, Warshawsky D. DMBA-induced mammary pathologies are angiogenic in vivo and in vitro. Lab Investig. 2000;80(4):485–492. doi: 10.1038/labinvest.3780054. [DOI] [PubMed] [Google Scholar]
- 28.Currier N, Solomon SE, Demicco EG, Chang DL, Farago M, Ying H, Dominguez I, Sonenshein GE, Cardiff RD, Xiao ZX, et al. Oncogenic signaling pathways activated in DMBA-induced mouse mammary tumors. Toxicol Pathol. 2005;33(6):726–737. doi: 10.1080/01926230500352226. [DOI] [PubMed] [Google Scholar]
- 29.Russo J, Russo IH. Biological and molecular bases of mammary carcinogenesis. Lab Investig. 1987;57(2):112–137. [PubMed] [Google Scholar]
- 30.Russo J, Gusterson BA, Rogers AE, Russo IH, Wellings SR, van Zwieten MJ. Comparative study of human and rat mammary tumorigenesis. Lab Investig. 1990;62(3):244–278. [PubMed] [Google Scholar]
- 31.Russo J, Russo IH. Atlas and histologic classification of tumors of the rat mammary gland. J Mammary Gland Biol Neoplasia. 2000;5(2):187–200. doi: 10.1023/a:1026443305758. [DOI] [PubMed] [Google Scholar]
- 32.Batcioglu K, Uyumlu AB, Satilmis B, Yildirim B, Yucel N, Demirtas H, Onkal R, Guzel RM, Djamgoz MB. Oxidative stress in the in vivo DMBA rat model of breast cancer: suppression by a voltage-gated sodium channel inhibitor (RS100642) Basic Clin Pharmacol Toxicol. 2012;111(2):137–141. doi: 10.1111/j.1742-7843.2012.00880.x. [DOI] [PubMed] [Google Scholar]
- 33.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 34.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244(22):6049–6055. [PubMed] [Google Scholar]
- 35.Catalase LH. Methods in enzymatic analysis. Weinhelm-New York: Verlag-Chemie-Academic Press; 1963. [Google Scholar]
- 36.Miki N, Nagano M, Kuriyama K. Catalase activates cerebral granylate cyclase in the presence of sodium azide. Biochem Biophys Res Commun. 1976;72(3):952–959. doi: 10.1016/s0006-291x(76)80224-0. [DOI] [PubMed] [Google Scholar]
- 37.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86(1):271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 38.Sastry KV, Moudgal RP, Mohan J, Tyagi JS, Rao GS. Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal Biochem. 2002;306(1):79–82. doi: 10.1006/abio.2002.5676. [DOI] [PubMed] [Google Scholar]
- 39.Schwertner HA, Rios DC. High-performance liquid chromatographic analysis of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol in ginger-containing dietary supplements, spices, teas, and beverages. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;856(1–2):41–47. doi: 10.1016/j.jchromb.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 40.Zhang CH, Yu Y, Liang YZ, Chen XQ. Purification, partial characterization and antioxidant activity of polysaccharides from Glycyrrhiza uralensis. Int J Biol Macromol. 2015;79:681–686. doi: 10.1016/j.ijbiomac.2015.05.060. [DOI] [PubMed] [Google Scholar]
- 41.Zong Y, Wang Y, Li H, Li N, Zhang H, Sun J, Niu X, Gao X. Simultaneous quantification and splenocyte-proliferating activities of nucleosides and bases in Cervi cornu Pantotrichum. Pharmacogn Mag. 2014;10(40):391–397. doi: 10.4103/0973-1296.141757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Wang R, An R, Wu X, Wang X, Li Y. Simultaneous determination of five constituents in Scrophularia ningpoensis by HPLC. Zhongguo Zhong Yao Za Zhi. 2011;36(6):709–711. [PubMed] [Google Scholar]
- 43.Seo CS, Shin HK. Simultaneous determination of nine marker compounds in the traditional Korean medicine, Dangguisu-san by high-performance liquid chromatography. Pharmacogn Mag. 2015;11(43):555–561. doi: 10.4103/0973-1296.160457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma R, Song G, You W, Yu L, Su W, Liao M, Zhang Y, Huang L, Zhang X, Yu T. Anti-microtubule activity of tubeimoside I and its colchicine binding site of tubulin. Cancer Chemother Pharmacol. 2008;62(4):559–568. doi: 10.1007/s00280-007-0635-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsao R, Yu Q, Potter J, Chiba M. Direct and simultaneous analysis of sinigrin and allyl isothiocyanate in mustard samples by high-performance liquid chromatography. J Agric Food Chem. 2002;50(17):4749–4753. doi: 10.1021/jf0200523. [DOI] [PubMed] [Google Scholar]
- 46.Kenney NJ, Smith GH, Lawrence E, Barrett JC, Salomon DS. Identification of stem cell units in the terminal end bud and Duct of the mouse mammary gland. J Biomed Biotechnol. 2001;1(3):133–143. doi: 10.1155/S1110724301000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gabay M, Li Y, Felsher DW. MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb Perspect Med. 2014;4:a014241. [DOI] [PMC free article] [PubMed]
- 48.Chung MW, Tsoutsman T, Semsarian C. Hypertrophic cardiomyopathy: from gene defect to clinical disease. Cell Res. 2003;13(1):9–20. doi: 10.1038/sj.cr.7290146. [DOI] [PubMed] [Google Scholar]
- 49.Altomonte M, Montagner R, Fonsatti E, Colizzi F, Cattarossi I, Brasoveanu LI, Nicotra MR, Cattelan A, Natali PG, Maio M. Expression and structural features of endoglin (CD105), a transforming growth factor beta1 and beta3 binding protein, in human melanoma. Br J Cancer. 1996;74(10):1586–1591. doi: 10.1038/bjc.1996.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 51.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dougher-Vermazen M, Hulmes JD, Bohlen P, Terman BI. Biological activity and phosphorylation sites of the bacterially expressed cytosolic domain of the KDR VEGF-receptor. Biochem Biophys Res Commun. 1994;205(1):728–738. doi: 10.1006/bbrc.1994.2726. [DOI] [PubMed] [Google Scholar]
- 53.Giri U, Sharma SD, Abdulla M, Athar M. Evidence that in situ generated reactive oxygen species act as a potent stage I tumor promoter in mouse skin. Biochem Biophys Res Commun. 1995;209(2):698–705. doi: 10.1006/bbrc.1995.1555. [DOI] [PubMed] [Google Scholar]
- 54.Kurokawa Y, Takamura N, Matsushima Y, Imazawa T, Hayashi Y. Studies on the promoting and complete carcinogenic activities of some oxidizing chemicals in skin carcinogenesis. Cancer Lett. 1984;24(3):299–304. doi: 10.1016/0304-3835(84)90026-0. [DOI] [PubMed] [Google Scholar]
- 55.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4(8):118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 57.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 58.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 59.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13(6):472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Weinberg RA. Mechanisms of malignant progression. Carcinogenesis. 2008;29(6):1092–1095. doi: 10.1093/carcin/bgn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sesti F, Tsitsilonis OE, Kotsinas A, Trougakos IP. Oxidative stress-mediated biomolecular damage and inflammation in tumorigenesis. In Vivo. 2012;26(3):395–402. [PubMed] [Google Scholar]
- 62.Xue T. Synergy in traditional Chinese medicine. Lancet Oncol. 2016;17(2):e39. doi: 10.1016/S1470-2045(15)00557-4. [DOI] [PubMed] [Google Scholar]
- 63.Zhou X, Seto SW, Chang D, Kiat H, Razmovski-Naumovski V, Chan K, Bensoussan A. Synergistic effects of Chinese herbal medicine: a comprehensive review of methodology and current research. Front Pharmacol. 2016;7:201. doi: 10.3389/fphar.2016.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jolad SD, Lantz RC, Chen GJ, Bates RB, Timmermann BN. Commercially processed dry ginger (Zingiber officinale): composition and effects on LPS-stimulated PGE2 production. Phytochemistry. 2005;66(13):1614–1635. doi: 10.1016/j.phytochem.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 65.Ling H, Yang H, Tan SH, Chui WK, Chew EH. 6-Shogaol, an active constituent of ginger, inhibits breast cancer cell invasion by reducing matrix metalloproteinase-9 expression via blockade of nuclear factor-kappaB activation. Br J Pharmacol. 2010;161(8):1763–1777. doi: 10.1111/j.1476-5381.2010.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan BS, Kang O, Mai CW, Tiong KH, Khoo AS, Pichika MR, Bradshaw TD, Leong CO. 6-Shogaol inhibits breast and colon cancer cell proliferation through activation of peroxisomal proliferator activated receptor gamma (PPARgamma) Cancer Lett. 2013;336(1):127–139. doi: 10.1016/j.canlet.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 67.Rhode J, Fogoros S, Zick S, Wahl H, Griffith KA, Huang J, Liu JR. Ginger inhibits cell growth and modulates angiogenic factors in ovarian cancer cells. BMC Complement Altern Med. 2007;7:44. doi: 10.1186/1472-6882-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu L, Hudgins WR, Shack S, Yin MQ, Samid D. Cinnamic acid: a natural product with potential use in cancer intervention. Int J Cancer. 1995;62(3):345–350. doi: 10.1002/ijc.2910620319. [DOI] [PubMed] [Google Scholar]
- 69.Hao W, Wang S, Zhou Z. Tubeimoside-1 (TBMS1) inhibits lung cancer cell growth and induces cells apoptosis through activation of MAPK-JNK pathway. Int J Clin Exp Pathol. 2015;8(10):12075–12083. [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y, Xu X, He P. Tubeimoside-1 inhibits proliferation and induces apoptosis by increasing the Bax to Bcl-2 ratio and decreasing COX-2 expression in lung cancer A549 cells. Mol Med Rep. 2011;4(1):25–29. doi: 10.3892/mmr.2010.379. [DOI] [PubMed] [Google Scholar]
- 71.Thejass P, Kuttan G. Inhibition of endothelial cell differentiation and proinflammatory cytokine production during angiogenesis by allyl isothiocyanate and phenyl isothiocyanate. Integr Cancer Ther. 2007;6(4):389–399. doi: 10.1177/1534735407309084. [DOI] [PubMed] [Google Scholar]
- 72.Xu H and Xu X. Polysaccharide, a Potential Anti-Cancer Drug with High Efficacy and Safety. J Oncol Res Treat. 2016;1:110.
- 73.Allard B, Beavis PA, Darcy PK, Stagg J. Immunosuppressive activities of adenosine in cancer. Curr Opin Pharmacol. 2016;29:7–16. doi: 10.1016/j.coph.2016.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed during the current study are available from the corresponding author on reasonable request.