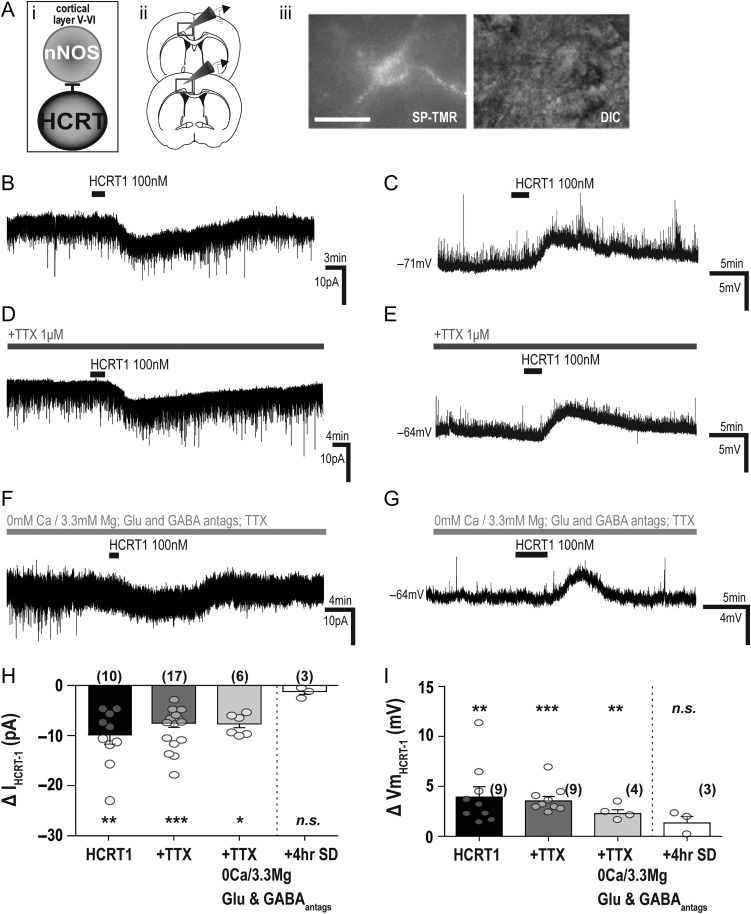
Figure 1.
Electrophysiological responses of cortical nNOS/NK1R cells to bath application of HCRT1/orexin-A. (Ai) Schematic illustrating experimental approach underlying in vitro electrophysiological studies of cortical nNOS/ NK1R cells (ii) in the deep layers of the cingulate cortex. (iii) Cortical nNOS/NK1R neurons were identified by application of SP-TMR, a fluorescent agonist for the NK1R (scale bar = 25 μm). (B) Bath application of hypocretin 1 (HCRT1; 100 nM) evoked an inward current and membrane depolarization (C). In the presence of tetrodotoxin (TTX; 1 μM), both the voltage-clamp (D) and current-clamp (E) responses persisted. To confirm a postsynaptic mechanism of action, HCRT1 was applied in the presence of glutamatergic (CNQX,7 μM; AP5, 100 μM) and GABAergic (2-HS, 5 μM; BIC, 10 μM) blockers, TTX and 0 Ca2+/3.3 Mg2+-containing aCSF (F–G). Summary of the HCRT1-evoked current (H) and membrane depolarization (I) on cortical nNOS/NK1R neurons. There were no statistically significant differences between HCRT1 application in normal aCSF, in the presence of TTX, or in 0 Ca2+/3.3 Mg2+-containing aCSF conditions in either voltage-clamp (1-way ANOVA, F(2,32) = 0.90, P = 0.41) or current-clamp conditions (1-way ANOVA, F(2,21) = 0.76, P = 0.48). However, after 4 h sleep deprivation, ex vivo cortical nNOS cells had no significant responses to HCRT1 application. The absence of a response was significantly different from that evoked in non-sleep-deprived mice in TTX-aCSF for current (unpaired t-test, t(18) = 2.90, P = 0.009) and membrane depolarization (unpaired t-test, t(10) = 2.49, P = 0.03).