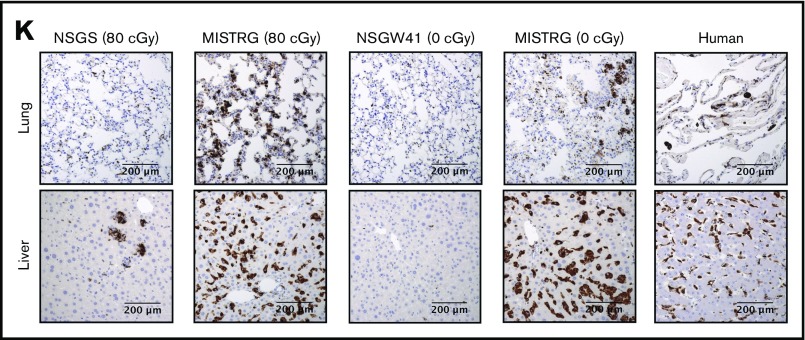
Figure 2.
NSGS, NSGW41, and MISTRG mice differentially support the development of human myeloid cell lineages. (A) Representative flow cytometry analysis and (B) frequency of human myeloid CD33+ cell populations in the BM of recipient mice 22 weeks after engraftment, distinguishing granulocytic (CD33+SSChi, blue gate) and monocytic (CD33hiSSClo, green gate) cells pregated on live 7-AAD–mTer119–mCD45–hCD45+ cells. (A) Numbers and (B) bars indicate frequencies among hCD45+ cells expressed as mean ± SD (NSGS mice, n = 4; irradiated MISTRG mice, n = 12; NSGW41 mice, n = 9; nonirradiated MISTRG mice: n = 9). (C) Representative flow cytometry analysis and (D) frequency of human myeloid CD33+ cell populations in the blood of recipient mice from panel A compared with human healthy donor blood (n = 6). (E) Representative flow cytometry analysis and (F) frequency of blood human neutrophils (CD66b+CD16+) within the CD33+/loSSChi population shown in the blue gate in panel C. The insets show representative images of sorted human CD33+/loSSChi granulocytic cells stained by Diff-Quik (scale bar: 20 µm). (G) Representative flow cytometry analysis and (H) frequency of human monocyte subsets (CD33hiSSClo, green gate in panel C further gated on CD117–FcεR1–CD203c– cells) in the blood of recipient mice 10 weeks after engraftment, as defined by CD14 and CD16 expression. (I) Representative images of sorted human CD33hiSSClo monocytes of the indicated CD14/CD16 phenotype stained by Diff-Quik (scale bar, 20 µm). (J) Frequency of cytokine-producing monocytes among human blood or MISTRG mouse blood cells measured by intracellular cell staining for tumor necrosis factor α (TNFα) and IL-6 after ex vivo stimulation with lipopolysaccharide (LPS; 100 ng/mL) or R848 (10 µg/mL) for 24 hours. (K) Tissue macrophages in the lungs and livers of recipient mice compared with normal human tissues, as identified by immunohistochemistry for human CD68. Histograms (B,D,F,H,J) represent mean ± SD.