Abstract
Cervical cancer continues to be a major public health problem affecting large numbers of women in many developing countries. Limitations of various screening modalities and the lack of ready availability of a cost-effective point-of-care screening tool have hindered the efficient implementation of population-based screening programs in these settings. It has not proved possible for many countries to adopt cytology as a screening modality due to inadequate infrastructure and trained manpower. However, recent developments, notably design and testing of a low-cost HPV test kit and initiatives by countries like India in developing and putting into operation a framework for large-scale screening of women, have raised hopes that cervical cancer control may be possible even in resource-constrained locations. With the advent of HPV vaccination, primary prevention of cervical cancer also seems a distinct possibility. However, wide availability and acceptability of vaccination is still an unresolved issue for developing countries. The possible future effects of vaccination on test characteristics of various screening strategies also need to be evaluated. This review gathers information on the current status of cervical cancer screening with a special focus on low resource settings. It revisits the strengths and limitations of the available screening modalities for cervical cancer viz. cytology, visual methods and HPV testing, in the context of their applicability in developing countries. In addition, the role of newer HPV-detection methods, for instance DNA, RNA and protein-based techniques, in triage of screen-positive women is discussed. The contemporary issue of impact of HPV vaccination on cervical cancer screening is also addressed briefly. The main highlight of the review is the reference to ‘operational framework guidelines’ for population-based cervical cancer screening, which have recently been formulated and are in the process of being implemented in India. The guidelines may serve as a model for other similar low-resource settings where implementation of cancer screening is desired.
Keywords: Cervical cancer, screening, low resource settings, HPV, vaccination
Introduction
Cervical cancer has been and continues to be one of the leading causes of cancer-related mortality in women across the globe. The last few decades have witnessed an uneven distribution in incidence of this cancer with more than 85% of the cases being detected in low and middle income countries (LMIC) (Jemal et al., 2011). The mortality of cervical cancer varies within LMIC from 1.6 per 100,000 women in western Asia to 22.5 per 100,000 in sub-Saharan Africa (Ferlay et al., 2015). On the other hand, the age-standardized incidence of cervical cancer in Europe and North America is less than 9 per 100,000 women (Curado et al., 2007). This imbalance is attributed mainly to the effective and successful implementation of organized cervical cancer screening programs in developed countries leading to early detection and appropriate management of precancerous lesions (Peto et al., 2004). Majority of resource-limited countries have not been able to effectively implement such screening programs due to various reasons such as lack of adequate infrastructure, trained manpower and absence of political will. The results on cervical cancer screening in the World Health Survey showed the mean crude coverage in developing countries to be 45% and effective coverage to be a dismal 19%. For instance, over 90% of women in Malawi, Ethiopia and Bangladesh reported never having had a pelvic exam. Hence, improving access to health system for women is mandatory, since this contact is a prerequisite for any screening program to succeed (Gakidou et al., 2008).
The main objectives of this review are to gather available data on cervical cancer screening in developed as well as developing nations, to identify feasible and cost-effective screening strategy for low-resource settings and to foresee the challenges in implementation of an effective cervical cancer screening program.
Figure 1.
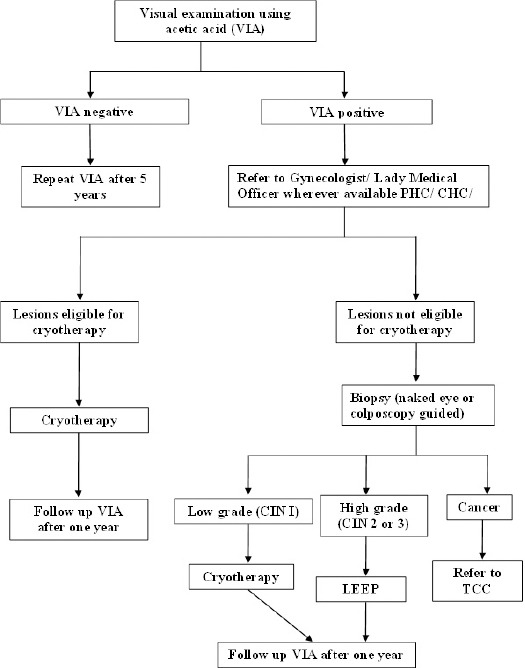
Algorithm for Screening and Management of Cervical Cancer in India (Operational Framework)
Cervical Cancer Screening Strategies in context of low-resource settings
The various methods currently available for cervical cancer screening include: cytology (Papanicolaou smear); visual methods (visual inspection with acetic acid (VIA) and Lugol’s iodine (VILI)); and HPV DNA-based detection tests. The merits and limitations of these methods are summarized in Table 1.
Table 1.
Overview of the Various Screening Modalities for Cervical Cancer
| Screening test | Strengths | Limitations |
|---|---|---|
| Cytology (Papanicolaou smear) | • High specificity for detection of CIN2+ lesions • Wide knowledge base and standardized reporting system • Relative low cost |
• Moderate sensitivity • Requires trained manpower • Delay in test results • Frequent revisits required (every 2-3 years) • Absence of adequate quality control |
| Visual methods (VIA/VILI) | • Requires less training • Less expensive and simple • Immediate results with option of “screen-and-treat” |
• Variable sensitivity and specificity • Likelihood of overtreatment • Inappropriate for older women • Lack of standardization of training and evaluation |
| HPV DNA-based test | • High sensitivity • High Negative Predictive Value • Requires minimal training • Self-collection of sample is possible |
• Lower specificity compared to cytology for CIN2+ • Cost-intensive • Requires laboratory setup • Delay in test results • Requires an additional test to detect CIN lesion |
Cervical cytology
Papanicolaou smear has been the cornerstone of well-organized population-based screening programs demonstrating remarkable success in reducing the incidence and mortality from cervical cancer in developed nations (Arbyn et al., 2009).
The World Health Organization has observed that incidence and mortality from cervical cancer can be significantly reduced by ensuring even once in a lifetime Pap smear-based screening of every eligible woman, ideally between 35-45 years of age (Stjernsward et al., 1987; WHO, 2002). The major strengths of cytology-based screening have been the inherent simplicity, relatively low cost and large knowledge base of various cytologic patterns of precancerous lesions (Tambouret, 2013). Though the sensitivity of cervical cytology is low, its specificity is quite high (60-95%) for detection of CIN2+ lesions (Nanda et al., 2000). Few recently published meta-analyses have also reaffirmed the high specificity and moderate sensitivity of cytology in detecting CIN2+ lesions as summarised in Table 2. The moderate sensitivity necessitates frequent testing to enable detection of precancerous lesion, which is usually problematic in low income countries for various logistic reasons (Cuzick et al., 2006). Despite the various operational limitations, few cervical cancer screening programs using cytology have been successful in resource-limited countries, such as “Viet/ American Cervical Cancer Prevention Project”, CerviCusco Clinic in Cusco, Peru and similar programs in Kenya and Zambia (Guzzetti et al., 2009; Hassan, 2010; Lin et al., 2011; Suba and Raab, 2012). These programs emphasize the potential success of screening programs utilizing local manpower trained in performance of screening tests.
Table 2.
Recent Published Meta-Analyses of Cytology as Cervical Cancer Screening Tool
| Author (Year) | Sensitivity, % (95% C.I.) | Specificity, % (95% C.I.) |
|---|---|---|
| Sankarnarayanan et al (2004a) | 61 (56-66) | 95 (94-95) |
| Kolipoulos et al (2007) | 61.6 | 96 |
| Cong Xeuyu et al (2007) | 60 (45-74) | 76 (66-76) |
| Chen et al (2012) | 59 | 94 |
| Chanthavilay et al (2015) | 62 (49-73) | 92 (78-97) |
| Li et al (2016) | 74.3 (71.6-76.8) | 95.1 (94.9-95.3) |
Attempts at improving the sensitivity and accuracy of cytology led to introduction of liquid-based cytology (LBC). LBC was aimed at reducing the unsatisfactory rate of cervico-vaginal cytology by providing a more representative sample and overcoming the limiting factors like mucus, blood and excessive inflammation. Various studies showed a significant increase in detection of LSIL or higher cytology on LBC compared to conventional cytology (Díaz-Rosario and Kabawat, 1999; Hatch et al., 2004; Lee et al., 1997). However, some recent studies have refuted any significant improvement in sensitivity over the conventional Pap test (Ronco et al., 2007). This combined with prohibitively high cost of the equipment and consumables renders this option less feasible for low-resource settings.
Visual inspection methods
The different visual inspection methods include unaided visual examination (‘down-staging’), visual inspection with acetic acid (VIA), visual inspection with Lugol’s iodine (VILI) and visual inspection with low-level magnification (VIAM) (Wright et al., 2002). Unaided visual examination has not been widely accepted or implemented in cervical cancer screening due to its low sensitivity. Over years, visual screening was supplemented by use of acetic acid (VIA) or Lugol’s iodine (VILI) to improve the sensitivity of visual detection. Various studies evaluating the test characteristics of VIA have demonstrated sensitivity ranging from 49-86% in cervical cancer screening (Shastri et al., 2005; Sodhani et al., 2006). Specificity of VIA has been reported to be similar to or slightly lower than cytology, supporting its potential utility in cervical cancer screening. A recent meta-analysis of alternative strategies for cervical cancer screening in sub-Saharan Africa showed a pooled sensitivity and specificity of VIA as 82.4% and 87.4%, respectively. The pooled sensitivity of VILI was higher than VIA (95.1%) but specificity was similar (87.2%). Lesions on VILI were less equivocal and easily recognized by healthcare providers leading to better performance of VILI (Sangwa-Lugoma et al., 2006). The results of few published meta-analyses on performance of VIA for detection of CIN2+ lesions are tabulated in Table 3.
Table 3.
Published Meta-Analyses of Studies Analyzing VIA as a Screening Tool
| Author (Year) | Sensitivity, % (95% C.I.) | Specificity, % (95% C.I.) |
|---|---|---|
| Sritipsukho P (2010) | 71.8 | 79.4 |
| Chen C et al (2012) | 77 | 87 |
| Chanthavilay P et al (2015) | 69 (57-79) | 76 (63-85) |
| Fokom-Domgue J et al (2015) | 82.4 (76.3-87.3) | 87.4 (77.1-93.4) |
Various benefits and limitations of visual detection methods are depicted in Table 1. Comprehensive competency-based training and regular assessment of test providers is mandatory to improve the specificity of VIA (Blumenthal et al., 2005). VIA administered by trained personnel for cervical cancer screening in over a lakh women in Bangladesh reaffirmed the sensitivity and specificity as 93.6% and 58.2%, respectively and hence, VIA was suggested as a screening tool for cervical cancer in Bangladesh (Nessa et al., 2010). The pilot cervical cancer screening program in two districts of Tamil Nadu implemented by World Bank-supported Tamil Nadu Health Systems Project (TNHSP) exemplifies a government-led prevention effort in a resource-limited setting like India. This project utilized VIA/ VILI for screening with referral to secondary level health services for further evaluation. The pilot project had a screening coverage of 73.8% in the target age group. About 56.5% of screen-positive women underwent colposcopy, of whom 7.5% had frank/ invasive carcinoma while 0.87% had CIN2/3 lesions (Tamil Nadu Health Systems Project). However, the screen positivity and follow-up rates were relatively low. An important issue highlighted relating to the project was the requirement of adequate consistent and high-quality training in interpreting VIA results with post-training knowledge and skill assessment. In spite of the limitations, TNHSP pilot has provided evidence for the feasibility and acceptability of cervical cancer prevention via public health system in presence of strong political and administrative support (Krishnan et al., 2013).
HPV-based testing
Since Human Papilloma Virus (HPV) has been causally linked with majority of the cervical cancers, numerous studies have evaluated HPV DNA testing, either as co-test with cytology or as a standalone primary cervical cancer screening tool. Currently, there are five FDA-approved assays for HPV DNA detection: Hybrid Capture 2 (13 HR-HPV types), Cervista HPVHR test (14 HR-HPV types), Cervista HPV 16/18, Cobas 4800 HPV test (PCR-based) and Aptima HPV (amplification-based) assay, (Stoler et al., 2011).
The main advantages of HPV testing are the high sensitivity (average 95%) ensuring low false-negative rate and a high negative predictive value allowing the screening interval to be extended safely in HR-HPV DNA negative women (Cuzick et al., 2008). Another potential advantage is the scope of self-collection of samples in order to improve acceptability and screening coverage, especially in low-resource settings. Bhatla et al reported the sensitivity and specificity of HPV-DNA detection for CIN2+ disease to be 82.5% and 93.6% respectively for self-collected samples compared to 87.5% and 93.2% for physician-collected samples (Bhatla et al., 2009). These advantages, however, come at the expense of relatively lower specificity. Shastri et al demonstrated HPV DNA testing to be more sensitive than cytology (62% vs 57.4%) but less specific (93.5% vs 98.6%) in detection of CIN2+ cytology (Shastri et al., 2005). A multicenter study in India showed sensitivity of HPV detection ranging from 45.7-80.9% and specificity between 91.7-94.6% compared to 36.6-72.3% sensitivity and 87.2-98.6% specificity for cytology (Sankarnarayanan et al., 2004b). The results of published meta-analyses of studies evaluating performance of HPV detection methods as cervical cancer screening tool are highlighted in Table 4.
Table 4.
Data of Published Meta-Analyses Evaluating HPV Testing in Cervical Cancer Screening
| Author | Sensitivity, % (95% C.I.) | Specificity, % (95% C.I.) |
|---|---|---|
| Koliopoulos et al (2007) | 90 | 86.5 |
| Chen C et al (2012) | 74 | 92 |
| Mustafa RA et al (2015) | 94 (89-97) | 88 (84-92) |
| Fokom-Domgue J et al (2015) | 88.3 (73.1-95.5) | 73.9 (50.7-88.7) |
The current international guidelines advocate the use of HPV DNA testing in a co-testing approach with cytology (Saslow et al., 2012), which combines the merits of high sensitivity of HPV DNA testing and better specificity of cytology. This enables an increase of the screening interval in women tested negative with both, without a significant risk of missing precancerous lesions. However, the cost of currently available HPV detection tests precludes the widespread utility of this method in cervical cancer screening in resource-poor settings. Recently, a cost-effective HPV test kit, CareHPV (Qiagen) has been evaluated in low-resource settings. Field evaluation in rural China showed the accuracy of Care HPV to be higher than VIA and approaching that of HC2 (Qiao et al., 2008). A multi-country evaluation of this test in India, Nicaragua and Uganda also confirmed the high sensitivity (81.5%, 76.5-85.8) and specificity (91.6%) of this test (Jeronimo et al., 2014). The commercial availability of this test is expected to allow wider applicability of HPV DNA testing in resource-limited countries.
Efficacy and Cost-effectiveness of screening modalities in low-resource settings
A cluster randomized controlled trial in India evaluated the efficacy and cost-effectiveness of a single screening round with VIA, cytology and HPV testing in the reducing the incidence and mortality from invasive cervical cancer in rural district. The trial demonstrated higher rates of detection of stage I cancer (53-67% in screened group compared to 19% in control arm) and significantly lower proportion of stage III cancers. The study concluded that VIA was a useful alternative to HPV testing for low-resource settings, provided standardized testing and careful monitoring of test positivity and detection rates are ensured (Sankaranarayanan et al., 2005). Cost-effectiveness of the three screening modalities has been evaluated in few studies. A cluster randomized controlled trial in rural India reported VIA to be the cheapest screening strategy at $3.92 per eligible woman followed by cytology at $6.6 and HPV detection at $11.8 per eligible female (Legood et al., 2005). Goldie et al used computer-based model to assess the cost-effectiveness of cervical cancer screening strategies in five developing countries, including India. The authors demonstrated that strategy of one-visit VIA was the least costly method in India costing about $10 per year of life saved while three-visit cytologic examination was more costly and less effective. An interesting finding was the effect of reduction of cost of HPV detection methods. If the cost of HPV DNA testing was reduced by 50%, two-visit HPV became the most cost-effective strategy overriding one-visit VIA and cost $1 per year of life saved for one screening, $73 for two screenings and $231 for three screenings (Goldie et al., 2005).
Emerging technologies and Biomarkers
Though HPV DNA tests demonstrate good performance in cervical cancer screening, these tests are not able to differentiate latent from transformative infection. Hence, technologies with ability to detect HPV-induced transformed lesions, such as DNA-based genotyping assays (Roche’s Linear Array® and Digene’s Luminex®-based HPV genotyping), RNA-based tests (APTIMA® (Gen-Probe), PreTect HPV-Proofer (Norchip, Norway) or NucliSENS Easy Q® (Bio-Mėrieux, France)) and protein-based assays (p16INK4a, Ki-67, P16/Ki67 dual staining, BD ProExC containing MCM2 and TOP2A proteins, interphase FISH for 3q26 gain detection; p63/p73 staining and PIK3CA) are continually being evaluated for utility in screening of cervical cancer or triage of screen-positive cases (Alameda et al., 2009; Cheung et al., 2010; Cuschieri and Wentzensen, 2008; Goto et al., 2006; Monsonego et al., 2011; Sahebali et al., 2003; Schmidt et al., 2011; Tambouret et al., 2008; van Hamont et al., 2006). However, the results of most of these assays have not been conclusive and hence, none of these have been recommended for screening or triage. The “ideal” biomolecular marker appropriate for cervical cancer screening still remains elusive.
HPV Vaccination Era: Status of cervical cancer screening
Till recently, cervical cancer control programs had focused on secondary prevention, i.e. screening and early detection of precancerous lesions. However, with the advent of HPV vaccines (Gardasil from Merck & Co. and Cervarix from Glaxo Smith-Kline), primary prevention of cervical cancer seems a distinct reality (World Health Organization 2007). Currently, majority of these vaccination programs target adolescent girls prior to onset of sexual activity (Schiffman and Wentzensen, 2013). Hence, it would take expectedly about 30 years to observe the benefits of vaccination once the vaccinated cohorts reach the peak age of occurrence of cervical cancer (Schiffman and Wentzensen, 2013). However, a significant decline in the rate of high-grade lesions is being reported from countries with ongoing vaccination program including “catch-up” age range of 13-26 years (Brotherton et al., 2011). The introduction of HPV vaccine in low resource countries has been limited by the high cost of the vaccine. However, efforts by GAVI, the Vaccine Alliance as well as the Gardasil Access Program in low and middle income countries have demonstrated the possibility of inclusion of HPV vaccination at lower cost through funding, provided a strong political and administrative will supports the program (Ladner et al., 2014; Levin et al., 2014). In India, two states- Delhi and Punjab have launched HPV vaccination recently (World Health Organization 2016). The results of efficacy of vaccination in prevention of cervical cancer shall be known in about three decades in these countries.
Since HPV vaccination is expected to result in significant reduction in cervical precancerous and cancerous lesions, a direct influence on the positive predictive value (PPV) of various screening tests is being speculated. Tota J et al, through their modelling techniques, hypothesized that vaccine-induced reduction in prevalence of precancerous changes would create a situation where cytology screening would not be cost-effective with PPV falling below 10% (Tota et al., 2014). The prevalence of squamous intraepithelial lesions attributable to HPV 16/18 is expected to decline leading to reduction in PPV (Tota et al., 2014). Also, decreased “signal-to-noise” ratio (fewer true squamous abnormalities in comparison to inflammation and reactive atypia) is likely to adversely affect the sensitivity and specificity of Pap test. However, a recent study showed a surprisingly small effect of HPV vaccination on PPV of cervical cytology screening (Franco et al., 2009). Large-scale prospective studies with strict quality control systems are required to confirm the effect of HPV vaccination on performance of cytology as a primary screening tool.
Though studies evaluating the efficacy of HPV testing in vaccinated women are still underway, it is proposed that primary HPV DNA testing with cytology triage would be efficacious combining the sensitivity of HPV and specificity of cytology (Tota et al., 2014). Apart from the expected cost-effectiveness of this algorithm, another benefit would be the “artificially-enriched” cytology case load maintaining the diagnostic accuracy of cytotechnologists (Palmer et al., 2016). Some additional issues that would need consideration in the post-vaccination era include the potential for HPV type replacement (other HPV types taking over after eradication of HPV 16/18) and the possibility of HPV vaccination promoting risky sexual behaviour leading to increase in other HPV genotypes, necessitating continual utility of cytology in screening or triage of HPV-positive women.
Future Directions: The “Operational Framework” model – a guidance document for cancer screening
There is a continuous search for the most appropriate and cost-effective strategy for population-based cervical cancer screening in low-resource countries. The international guidelines (American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP) and American Society for Clinical Pathology (ASCP)) advocate initiation of cervical cancer screening at the age of 21 years using cytology every 3 years till 30 years of age. Beyond 30 years of age, co-testing with HPV and cytology every 5 years is recommended (Saslow et al., 2012). These, however, are not applicable in most of the low-resource settings due to various constraints. An appropriate screening algorithm for low-resource countries may include low cost HPV-based testing (as primary or co-testing approach) or VIA if financial constraints prohibit the use of HPV DNA testing.
Recently, the Ministry of Health and Family Welfare of India, a resource-limited country, formulated the screening and management algorithm for population-based screening of cervical cancer along with cancers of breast and oral cavity, the three most common cancer sites in India. The framework proposes institution of awareness and screening activities at the subcentre level, i.e. closest to the population to be screened. It advocates VIA to be used as a screening tool for cervical cancer screening with linkages at higher levels to ensure high quality treatment at affordable costs with regular follow-up. The implementation of this program is intended to be through the existing health system supported by the District NCD (Non-communicable disease) Cell for planning, monitoring and reporting. The states have been given opportunity to implement the program in a phased manner. The sub-centers and primary health centres of the selected districts are to be developed as cancer screening centres and equipped to provide basic services as well as awareness activities. District Hospital and Community Health Centres (CHC) would be equipped for confirmation of screen-positive cases as well as first line management of cases. These centres, in turn, shall be linked to the nearest tertiary level health facility for referral and follow-up as also for mentoring and support. Adequate training and retraining to build competencies at each level of health care facility would be ensured for successful running of the program (Operational Framework. Management of Common Cancers). The ‘operational framework’ is a guidance document which other resource-constrained countries desirous of implementing cervical cancer screening can replicate with necessary adaptations.
There are still a lot of unanswered questions and challenges ahead viz. determining appropriate cost-effective and point of care screening strategy, deciding intervals of screening, motivation of women to come forward for screening with optimal utilization and augmentation of existing health care infrastructure including diagnostic and treatment facilities. The issues related to implementation of HPV vaccination and applicability of novel biomarkers in triage of HPV-positive women also need to be further explored. The ongoing cervical cancer screening trials, in both developed and developing countries shall address some of these issues. The commercial availability of low-cost HPV DNA test is also expected to boost the efforts of definition of the appropriate screening algorithm for resource-limited countries. The triad of strong political will, judicious allocation of finances, and strategic communication would be the important keys to the success of cancer prevention programming in resource constrained settings.
Conflict of Interest
The authors declare no conflict of interests.
References
- Alameda F, Espinet B, Corzo C, et al. 3q26 (hTERC) gain studied by fluorescence in situ hybridization as a persistence-progression indicator in low-grade squamous intraepithelial lesion cases. Hum Pathol. 2009;40:1474–8. doi: 10.1016/j.humpath.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Arbyn M, Raifu AO, Weiderpass E, Bray F, Anttila A. Trends of cervical cancer mortality in the member states of the European Union. Eur J Cancer. 2009;45:2640–8. doi: 10.1016/j.ejca.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Bhatla N, Dar L, Patro AR, et al. Can human papillomavirus DNA testing of self-collected samples compare with physician-collected cervical samples and cytology for cervical cancer screening in developing countries? Cancer Epidemiol. 2009;33:446–50. doi: 10.1016/j.canep.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal PD, Lauterbach M, Sellors JW, Sankaranarayanan R. Training for cervical cancer prevention programs in low-resource settings: focus on visual inspection with acetic acid and cryotherapy. Int J Gynaecol Obstet. 2005;89:30–7. doi: 10.1016/j.ijgo.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Brotherton JM, Fridman M, May CL, et al. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet. 2011;377:2085–92. doi: 10.1016/S0140-6736(11)60551-5. [DOI] [PubMed] [Google Scholar]
- Chanthavilay P, Mayxay M, Phongsavan K, et al. Accuracy of combined visual Inspection with Acetic Acid and cervical cytology testing as a primary screening tool for cervical cancer: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2015;16:5889–97. doi: 10.7314/apjcp.2015.16.14.5889. [DOI] [PubMed] [Google Scholar]
- Chen C, Yang Z, Li Z, Li L. Accuracy of several cervical screening strategies for early detection of cervical cancer: a meta-analysis. Int J Gynecol Cancer. 2012;22:908–21. doi: 10.1097/IGC.0b013e318256e5e4. [DOI] [PubMed] [Google Scholar]
- Cheung AN, Tsun KL, Ng KM, et al. P634A4 and TAp73 immunocytochemistry in liquid-based cervical cytology-potential biomarkers for diagnosis and progress prediction of cervical neoplasia. Mod Pathol. 2010;23:559–66. doi: 10.1038/modpathol.2009.198. [DOI] [PubMed] [Google Scholar]
- Cong X, Cox DD, Cantor SB. Bayesian meta-analysis of Papanicolaou smear accuracy. Gynecol Oncol. 2007;107:133–7. doi: 10.1016/j.ygyno.2007.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curado MP, Edwards B, Shin HR, et al. Cancer incidence in five continents. No. 160. IX. Lyon, France: IARC Press; 2007. [Google Scholar]
- Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2008;17:2536–45. doi: 10.1158/1055-9965.EPI-08-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095–101. doi: 10.1002/ijc.21955. [DOI] [PubMed] [Google Scholar]
- Cuzick J, Arbyn M, Sankaranarayanan R, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008;26:29–41. doi: 10.1016/j.vaccine.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Díaz-Rosario LA, Kabawat SE. Performance of a fluid-based, thin-layer papanicolaou smear method in the clinical setting of an independent laboratory and an outpatient screening population in New England. Arch Pathol Lab Med. 1999;123:817–21. doi: 10.5858/1999-123-0817-POAFBT. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Fokom-Domgue J, Combescure C, Fokom-Defo V, et al. Performance of alternative strategies for primary cervical cancer screening in sub-Saharan Africa: systematic review and meta-analysis of diagnostic test accuracy studies. BMJ. 2015;351:h3084. doi: 10.1136/bmj.h3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco EL, Mahmud SM, Tota J, Ferenczy A, Coutlée F. The expected impact of HPV vaccination on the accuracy of cervical cancer screening: the need for a paradigm change. Arch Med Res. 2009;40:478–85. doi: 10.1016/j.arcmed.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Gakidou E, Nordhagen S, Obermeyer Z. Coverage of cervical cancer screening in 57 countries: low average levels and large inequalities. PLoS Med. 2008;5:e132. doi: 10.1371/journal.pmed.0050132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, et al. Cost-effectiveness of cervical cancer screening in five developing countries. N Engl J Med. 2005;353:2158–68. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- Goto T, Takano M, Sasa H, et al. Clinical significance of immunocytochemistry for PIK3CA as a carcinogenesis-related marker on liquid-based cytology in cervical intraepithelial neoplasia. Oncol Rep. 2006;15:387–91. [PubMed] [Google Scholar]
- Guzzetti S, Sugrue C, Viberti L, et al. Telepathology as a service support for pap test diagnosis in Zambia: the experience of pathology beyond borders. Cancer Cytopathol. 2009;117:352. [Google Scholar]
- Hassan N The cervi cusco project. The bulletin. XLVII. Wilmington DE: American society of cytopathology; 2010. pp. 154–6. [Google Scholar]
- Hatch KD, Sheets E, Kennedy A, et al. Multicenter direct to vial evaluation of a liquid-based pap test. J Low Genit Tract Dis. 2004;8:308–12. doi: 10.1097/00128360-200410000-00009. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jeronimo J, Bansil P, Lim J, et al. A multicountry evaluation of careHPV testing, visual inspection with acetic acid, and papanicolaou testing for the detection of cervical cancer. Int J Gynecol Cancer. 2014;24:576–85. doi: 10.1097/IGC.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliopoulos G, Arbyn M, Martin-Hirsch P, et al. Diagnostic accuracy of human papillomavirus testing in primary cervical screening: a systematic review and meta-analysis of non-randomized studies. Gynecol Oncol. 2007;104:232–46. doi: 10.1016/j.ygyno.2006.08.053. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Madsen E, Porterfield D, Varghese B. Advancing cervical cancer prevention in India: implementation science priorities. Oncologist. 2013;18:1285–97. doi: 10.1634/theoncologist.2013-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladner J, Besson M-H, Rodrigues M, Audureau E, Saba J. Performance of 21 HPV vaccination programs implemented in low and middle-income countries 2009–2013. BMC Public Health. 2014;14:670. doi: 10.1186/1471-2458-14-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KR, Ashfaq R, Birdsong GG, et al. Comparison of conventional Papanicolaou smears and a fluid-based, thin-layer system for cervical cancer screening. Obstet Gynecol. 1997;90:278–84. doi: 10.1016/S0029-7844(97)00228-7. [DOI] [PubMed] [Google Scholar]
- Legood R, Gray AM, Mahé C, et al. Screening for cervical cancer in India: How much will it cost? A trial based analysis of the cost per case detected. Int J Cancer. 2005;117:981–7. doi: 10.1002/ijc.21220. [DOI] [PubMed] [Google Scholar]
- Levin A, Wang SA, Levin C, Tsu V, Hutubessy R. Costs of introducing and delivering HPV vaccines in low and lower middle income countries: Inputs for GAVI policy on introduction grant support to countries. PLoS One. 2014;9:e101114. doi: 10.1371/journal.pone.0101114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Li Y, Yang GX, et al. Diagnostic value of combination of HPV testing and cytology as compared to isolated cytology in screening cervical cancer: A meta-analysis. J Cancer Res Ther. 2016;12:283–9. doi: 10.4103/0973-1482.154032. [DOI] [PubMed] [Google Scholar]
- Lin Y, Matsuo K, Yoo E, et al. Clinical performance of a self-sustaining cervical cancer screening clinic in Nairobi, Kenya: the PAPS team experience. Gynecol Oncol. 2011;120:121–2. [Google Scholar]
- Monsonego J, Hudgens MG, Zerat L, et al. Evaluation of oncogenic human papillomavirus RNA and DNA tests with liquid-based cytology in primary cervical cancer screening: the FASE study. Int J Cancer. 2011;129:691–701. doi: 10.1002/ijc.25726. [DOI] [PubMed] [Google Scholar]
- Mustafa RA, Santesso N, Khatib R, et al. Systematic reviews and meta-analyses of the accuracy of HPV tests, visual inspection with acetic acid, cytology, and colposcopy. Int J Gynaecol Obstet. 2016;132:259–65. doi: 10.1016/j.ijgo.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Nanda K, McCrory DC, Myers ER, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000;132:810–9. doi: 10.7326/0003-4819-132-10-200005160-00009. [DOI] [PubMed] [Google Scholar]
- Nessa A, Hussain MA, Rahman JN, et al. Screening for cervical neoplasia in Bangladesh using visual inspection with acetic acid. Int J Gynaecol Obstet. 2010;111:115–8. doi: 10.1016/j.ijgo.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Operational framework. Management of common cancers. 2015. available from http://nicpr.res.in/images/PDF/Operational_Framework_Management_of_Common_Cancers.pdf .
- Palmer TJ, McFadden M, Pollock KG, et al. HPV immunisation and cervical screening--confirmation of changed performance of cytology as a screening test in immunised women: a retrospective population-based cohort study. Br J Cancer. 2016;114:582–9. doi: 10.1038/bjc.2015.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet. 2004;364:249–56. doi: 10.1016/S0140-6736(04)16674-9. [DOI] [PubMed] [Google Scholar]
- Qiao YL, Sellors JW, Eder PS, et al. A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. Lancet Oncol. 2008;9:929–36. doi: 10.1016/S1470-2045(08)70210-9. [DOI] [PubMed] [Google Scholar]
- Ronco G, Cuzick J, Pierotti P, et al. Accuracy of liquid based versus conventional cytology: overall results of new technologies for cervical cancer screening: randomised controlled trial. BMJ. 2007;335:28. doi: 10.1136/bmj.39196.740995.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahebali S, Depuydt CE, Segers K, et al. Ki-67 immunocytochemistry in liquid based cervical cytology: useful as an adjunctive tool? J Clin Pathol. 2003;56:681–6. doi: 10.1136/jcp.56.9.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwa-Lugoma G, Mahmud S, Nasr SH, et al. Visual inspection as a cervical cancer screening method in a primary health care setting in Africa. Int J Cancer. 2006;119:1389–95. doi: 10.1002/ijc.21972. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R, Basu P, Wesley RS, et al. Accuracy of visual screening for cervical neoplasia: Results from an IARC multicenter study in India and Africa. Int J Cancer. 2004a;110:907–13. doi: 10.1002/ijc.20190. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R, Chatterji R, Shastri SS, et al. Accuracy of human papillomavirus testing in primary screening of cervical neoplasia: results from a multicenter study in India. Int J Cancer. 2004b;112:341–7. doi: 10.1002/ijc.20396. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R, Nene BM, Dinshaw KA, et al. A cluster randomized controlled trial of visual, cytology and human papillomavirus screening for cancer of the cervix in rural India. Int J Cancer. 2005;116:617–23. doi: 10.1002/ijc.21050. [DOI] [PubMed] [Google Scholar]
- Saslow D, Solomon D, Lawson HW, et al. American cancer society, American society for colposcopy and cervical pathology, and American society for clinical pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147–72. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman M, Wentzensen N. Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:553–60. doi: 10.1158/1055-9965.EPI-12-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, Bergeron C, Denton KJ, Ridder R. European CINtec cytology study group p16/ki-67 dual-stain cytology in the triage of ASCUS and LSIL papanicolaou cytology: results from the European equivocal or mildly abnormal Papanicolaou cytology study. Cancer Cytopathol. 2011;119:158–66. doi: 10.1002/cncy.20140. [DOI] [PubMed] [Google Scholar]
- Shastri SS, Dinshaw K, Amin G, et al. Concurrent evaluation of visual, cytological and HPV testing as screening methods for the early detection of cervical neoplasia in Mumbai, India. Bull World Health Organ. 2005;83:186–94. [PMC free article] [PubMed] [Google Scholar]
- Sodhani P, Gupta S, Sharma JK, et al. Test characteristics of various screening modalities for cervical cancer: a feasibility study to develop an alternative strategy for resource-limited settings. Cytopathology. 2006;17:348–52. doi: 10.1111/j.1365-2303.2006.00351.x. [DOI] [PubMed] [Google Scholar]
- Sritipsukho P, Thaweekul Y. Accuracy of visual inspection with acetic acid (VIA) for cervical cancer screening: a systematic review. J Med Assoc Thai. 2010;93:254–61. [PubMed] [Google Scholar]
- Stjernsward J, Eddy D, Luthra U, Stanley K. Plotting a new course for cervical cancer screening in developing countries. World Health Forum. 1987;8:42–5. [Google Scholar]
- Stoler MH, Wright TC, Jr, Sharma A, et al. High-risk human papillomavirus testing in women with ASC-US cytology: results from the ATHENA HPV study. Am J Clin Pathol. 2011;135:468–75. doi: 10.1309/AJCPZ5JY6FCVNMOT. [DOI] [PubMed] [Google Scholar]
- Suba EJ, Raab SS. Lessons learned from successful Papanicolaou cytology cervical cancer prevention in the Socialist Republic of Vietnam. Diagn Cytopathol. 2012;40:355–66. doi: 10.1002/dc.21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambouret RH, Misdraji J, Wilbur DC. Longitudinal clinical evaluation of a novel antibody cocktail for detection of high-grade squamous intraepithelial lesions on cervical cytology specimens. Arch Pathol Lab Med. 2008;132:918–25. doi: 10.5858/2008-132-918-LCEOAN. [DOI] [PubMed] [Google Scholar]
- Tambouret R. Screening for cervical cancer in low-resource settings in 2011. Arch Pathol Lab Med. 2013;137:782–90. doi: 10.5858/arpa.2011-0695-RA. [DOI] [PubMed] [Google Scholar]
- Tamil Nadu health systems project. Prevention and care for women: Cervical cancer screening pilot program. Available at http://www.tnhsp.org/files/cervical%20cancer.pdf .
- Tota JE, Ramana-Kumar AV, El-Khatib Z, Franco EL. The road ahead for cervical cancer prevention and control. Curr Oncol. 2014;21:e255–64. doi: 10.3747/co.21.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hamont D, van Ham MA, Bakkers JM, Massuger LF, Melchers WJ. Evaluation of the SPF10-INNO LiPA human papillomavirus (HPV) genotyping test and the roche linear array HPV genotyping test. J Clin Microbiol. 2006;44:3122–9. doi: 10.1128/JCM.00517-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World health organization. National cancer control programs: policies and managerial guidelines (Second Edition) Geneva, Switzerland: WHO; 2002. [PubMed] [Google Scholar]
- World health organization. Meeting of the immunization strategic advisory group of experts, Nov 2006 - conclusions and recommendations. Wkly Epidemiol Rec. 2007;82:1–16. Available at http://www.who.int/wer/2007/wer8201-02.pdf . [PubMed] [Google Scholar]
- World health organization. Punjab launches HPV vaccine with WHO support. 2016. Available at http://searo.who.int/india/mediacentre/events/2016/Punjab_HPV_vaccine/en/
- Wright TC, Jr, Menton M, Myrtle JF, Chow C, Singer A. Visualization techniques (colposcopy, direct visual inspection, and spectroscopic and other visual methods). Summary of task force 7. Acta Cytol. 2002;46:793–800. doi: 10.1159/000327049. [DOI] [PubMed] [Google Scholar]


