Abstract
Background:
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common malignancy that is associated with high morbidity and mortality. Salivary lactate dehydrogenase (LDH concentration), as an expression of cellular necrosis, may be a special marker of lesions that occur with changes in the integrity of the oral mucosa. This study was performed to determine the accuracy of salivary LDH as a clinical marker for HNSCC detection and to investigate the relationship between salivary LDH levels and tissue tumor detection.
Methods:
The case group consisted of 44 HNSCC patients and the control group consisted of 44 healthy subjects. The stage and grade of HNSCC were determined, and the LDH levels in collected saliva samples were measured in all subjects. The expression of LDH in tumors and healthy tissue margins was evaluated via immunohistochemistry.
Results:
The expression of LDH in the saliva of patients with HNSCC is significantly higher than that in the saliva of the healthy control group. The expression of salivary LDH in patients with oral squamous cell carcinoma (OSCC) is significantly higher than that in the other patients and healthy individuals in the control group. The levels of salivary LDH in patients with SCC of the tongue and lower oral cavity were significantly higher than those in other patients affected with SCC in other parts of the head and neck (P<0.01).
Conclusion:
As this enzyme increases simultaneously in both tumoral tissues and saliva, it can serve as a useful diagnostic marker for the early diagnosis and prediction of HNSCC.
Key Words: Biomarker , Early diagnosis, Head and neck squamous cell carcinoma (HNSCC), Lactate dehydrogenase (LDH), Saliva
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the most common head and neck cancer and the sixth most common cancer globally, with over 650,000 new cases of the disease occurring every year all over the world. HNSCC arises from the mucosal lining of the oral cavity, sinonasal tract, nasopharynx, larynx, pharynx, oropharynx, and hypopharynx (1-5). Its major risk and promotive factors are human papilloma virus infection and the use of alcohol and tobacco (6-8). It is a disease associated with major morbidity and mortality, which increase with delay in diagnosis and inadequate response to tumor treatment. The single most effective factor in determining the prognosis of HNSCC is early and accurate diagnosis. In cancer diagnosis, essential tools are salivary and serum biomarkers. The use of saliva as a means of diagnosing cancer has become a popular approach in the last few years because the method is non-invasive and economical (9-12).
There are five active human tetrameric lactate dehydrogenase (LDH) isoenzymes, each of which composes two major subunits, A and B, which are encoded by LDH-A and LDH-B genes. When more A chains than B chains exist, LDH isoenzymes become more efficient in the catalytic conversion of pyruvate to lactate. Conversely, a greater number of B chains facilitates the conversion of pyruvate into acetyl coenzyme A (13-15). Sometimes, cells are emptied of glucose and release atomic carbon as carbon dioxide and atomic hydrogen as water. The energy production pipeline stops at the end of glycolysis under insufficient oxygen flow-a cellular problem that can be addressed by LDH. In this manner, LDH acts a “safe valve” in energy production. With cell dysplastic transformation, the tendency to use the anaerobic phase increases the glycolytic pathway (13-15).
Cancer cells are dependent on several metabolic processes, including glycolysis, glutaminolysis, and mitochondrial oxidative phosphorylation (OXPHOS), in satisfying their energy needs. Similar to most aggressive tumors, HNSCC activates glycolysis to a high degree for the purpose of meeting the metabolic demands of the cancer cells (16, 17). The glycolysis pathway serves as the foundation for cellular metabolism. In the regulation of glycolysis enzymes and glucose transporters, cells produce two adenosine triphosphates (ATPs). The tumor suppressors regulate the activity of glycolysis, thereby they can inhibit the growth of tumor cells; in contrast, the oncogenes can lead to an increase in the ATP levels through the aerobic glycolysis pathway. However, mitochondrial respiration can produce 36 ATPs by using a product of glycolysis-pyruvate-through OXPHOS. Glycolysis is the main characteristic of cancer cell metabolism. Although glycolysis produces less energy than do ATPs and OXPHOS, cancer cells generate fewer ATPs through OXPHOS because of weakened OXPHOS and the reduced use of pyruvate. Cancer cells aggressively activate glycolysis for energy balance. During glycolysis, the fast production of energy elevates cellular increase in quickly growing cancer cells, including HNSCC cells. In cancer cells, increased LDH catalyzes most pyruvates and causes them to lactate (10, 17-20).
Diagnostic biomarkers are currently used for the early detection of numerous types of lesions. In various studies, salivary biomarkers have been employed as non-invasive tools for diagnosing cancerous and premalignant lesions. With the improvement of knowledge on this issue, we hope to establish a target therapy and achieve advanced therapeutic and diagnostic aims. In this study, therefore, we correlated the expression of LDH in the saliva of HNSCC patients with the expression of this enzyme in the exponent biopsy of tumoral tissues and healthy tissue margins. The association of salivary LDH with tissue LDH expression may have important prognostic and therapeutic implications for HNSCC patients.
Materials and Methods
Study population
A comparative study was performed on two groups, namely, 44 HNSCC patients (case or patient group) and 44 healthy subjects (control group), each having 11 women and 33 men. The participants were referred to the Otorhinolaryngology Department of the Mashhad University of Medical Sciences between May 2014 and September 2017. The control group was matched with the case group in terms of age (±3 years) and sex. Patients who have undergone surgery, chemotherapy, and radiotherapy for their cancer and patients with controlled systemic diseases that may increase serum LDH levels and other conditions in the mouth that elevate LDH concentrations in saliva (e.g., periodontitis and mucosal lesions with tissue destruction) were excluded from the study. This study was approved by the Ethics Committee of the Mashhad University of Medical Sciences, and informed consent was received from all the patients.
Saliva collection and determination of LDH expression in saliva
Samples were preferentially collected in the morning to avoid daily salivary biochemical changes. The patients were prohibited from consuming water or food for an hour before sampling to prevent food and water interference in salivary enzyme levels. Whole saliva samples were collected through the spitting technique. The patients were requested to sit in a comfortable place and gargle their mouth with 30ml of pure water. Then, they were asked to collect 2ml of their saliva within 5 minutes in a sterilized micro tube. The saliva specimens were stored in an ice box and immediately transferred to a laboratory before being centrifuged for 5–7 minutes at 2000 rpm. Supernatant was collected for testing. Subsequently, LDH measurement steps were performed on the samples. LDH enzyme concentrations in the saliva of the case and control groups were measured via enzyme-linked immunosorbent assay using a human lactate dehydrogenase kit (abx053056, Abbexa, United Kingdom), according to the manufacturer’s protocols.
Tissue samples
The patients were fully examined, and their demographic status was recorded. Tumor tissues and healthy tissue margin samples were obtained from them. Fresh tissue samples were fixed in 10% formalin, after which they were subjected to formalin fixation and paraffin embedding. Tissue sections with a thickness of 4 µm were cut on a microtome for histopathological grading using routine hematoxylin and eosin staining. Patients with a histopathological diagnosis of HNSCC were classified on the basis of tumor grade into well-differentiated, moderately differentiated, and poorly differentiated groups using Broder’s grading of tumor differentiation (Fig. 1) (21).
Fig. 1.
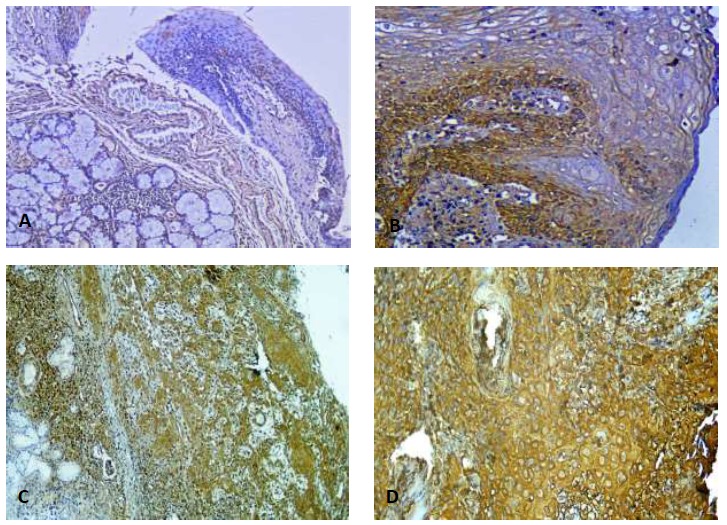
Immunohistochemistry of LDH expression in tumor tissues and healthy tissue margins. Patients exhibited significantly different expressions of LDH in negative and positive cancer tissues. A: In the high power field (100%) at 3+ intensity, basal layer cells are positively stained, whereas squamous layer cells are negatively stained. B: 90% cytoplasmic expression of LDH in basal layer cells, with no expression in superficial levels (200X magnification). C: 90% cytoplasmic expression of LDH with 2+ intensity at grade II SCC. D: Cytoplasmic expression of LDH in tumoral tissues with 3+ intensity and 100% cellular expression.
Immunohistochemical testing following tissue staining
Tissue sections of 3 mm thickness were placed onto slides coated with poly-lysine and incubated at 37 °C overnight and at 60 °C for an additional hour before staining. The slides were dewaxed with saline sodium citrate (pH=7.0) and incubated in this solution for 1 minute in a pressure heating environment for antigen retrieval. The slides were then soaked in 3% hydrogen peroxide solution for 30 minutes. Mouse monoclonal antibody (Santa Cruz Biotechnology, LDH (H-10): sc-133123) was applied at 4 °C overnight (1:200 dilution, sc-133123AF647). This antibody is recommended for use in the detection of LDH-A, LDH-B, and LDH-C of human origin. A secondary antibody was added to the slides, which were then left at room temperature for 25 minutes. Color was developed with diaminobenzidine. Two pathologists independently examined the stained specimens. To calculate the percentage of positive cells, at least five fields of view were observed for each section under a lens (200 X). The cytoplasmic expression of the studied marker was evaluated in terms of cellularity and intensity. The cellularity expression was uniform in staining from 0 to 100%, and the intensity of staining was scored accordingly: no staining (0), weak staining (+1), moderate staining (+2) and strong staining (+3) (22).
Statistical methods
The Statistical Package for the Social Sciences SPSS (version 11.5) was used for data analysis. The levels of LDH expression were compared in the tumoral and normal tissues of the patients, as well as in saliva of the patients and control group matched for age and gender using independent samples t-test. One way ANOVA test was used to examine the level of LDH expression in tumoral and normal tissues and its relationship with clinicopathological parameters and also to assess the relationship of LDH levels between saliva and tumor. Pearson’s correlation coefficient was used to investigate the correlation of LDH expression level in saliva with tissue. A p-value of 0.05 was considered statistically significant.
Results
Patients and clinical characteristics
The median age of the HNSCC patients was 59.61±13.22 (ranging from 27 to 83 years). Their clinicopathological characteristics are shown in Table 1. The results on the relationship between stage and histological grade and LDH expression in saliva and tissue are also presented in Table 1. As shown in the table, 61.4% (27/44) of the cases were well-differentiated, 31.8% (14/44) were moderately differentiated, and 6.8% (3/44) were poorly differentiated.
Table 1.
Associations between clinicopathological indexes and saliva and tissue LDH expression levels (intensity and cellularity).
| Characteristics | No | Percentage of cells in tumor tissues with LDH expression (%) | p value | Intensity of LDH expression in tumor tissues | p value | Salivary LDH (ng/mL) | p value | |
|---|---|---|---|---|---|---|---|---|
| Age | ≤59 | 23 | 70.20±14.30 | 0.47 | 2.13±0.63 | 0.53 | 294.69±68.33 | 0.68 |
| >59 | 21 | 74.76±17.35 | 2.33±0.55 | 242.00±94.62 | ||||
| Gender | Male | 33 | 70.61±18.86 | 0.57 | 2.18±0.68 | 0.51 | 246.39±90.05 | 0.01 |
| Female | 11 | 77.27±10.09 | 2.36±0.51 | 340.45±109.21 | ||||
| Tumor stage | Early | 19 | 70.00±15.27 | 0.45 | 2.26±0.65 | 0.75 | 271.26±96.53 | 0.94 |
| Advanced | 25 | 74.00±18.70 | 2.20±0.64 | 268.88±108.76 | ||||
| Histological grade | I | 27 | 75.56±14.76 | 0.04 | 2.33±0.68 | 0.19 | 279.93±109.20 | 0.72 |
| II | 14 | 70.71±18.59 | 2.14±0.53 | 255.64±88.82 | ||||
| III | 3 | 50.00±20.22 | 1.67±0.68 | 246.33±126.29 | ||||
| Tumor position | Lip | 10 | 78.00±19.32 | 0.49 | 2.50± 0.71 | 0.34 | 242.40±37.607 | 0.001 |
| Tongue | 14 | 72.14±19.29 | 2.21±0.69 | 388.50±92.022 | ||||
| Larynx | 16 | 66.88±14.48 | 2.00±0.52 | 201.62±39.007 | ||||
| Nasal | 2 | 81.00±14.14 | 2.50±0.71 | 225.00±49.497 | ||||
| Ear | 2 | 80.00±14.14 | 2.50±0.71 | 168.50±0.707 | ||||
LDH expression in tumoral tissues and healthy tissue margins
Both HNSCC tissues and healthy tissue margins were taken from each patient, and the samples from both groups were stained under the same conditions (Fig. 1). Independent samples t-test was performed to examine the relationship between LDH expression in the HNSCC and healthy tissues. The average percentage of LDH expression in tumor cells and healthy tissues was significant (P<0.001). On the basis of the above-mentioned scoring system, 34.1% of the cases (15/44) showed strong staining (score3), 54.5% (24/44) exhibited medium staining (score 2), and 11.4% (5/44) reflected weakly reactive staining (score 1), regardless of the histological grade of the patient groups (Fig. 1). Among the healthy tissue margins, 4.5% (2/44) showed strong staining, 40.9% (18/44) showed medium staining, and 54.5% (24/44) were weakly reactive (Fig. 1). The mean percentage of cells showing LDH expression in the tumor tissues was 72%±17, and the percentage of LDH expression in the healthy margin around a tumor was 43%±21 (P<0.001). A significant correlation [Pearson’s correlation: r=0.759; P<0.001] was found between the percentage of cells that showed LDH expression in tumors with the intensity of LDH expression in tumor tissues. A significant correlation (r=0.515; P<0.001) was also found between the percentage of cells that showed LDH expression in tumor tissues and the percentage of cells that showed LDH expression in healthy tissue margins.
Salivary LDH expression in patients and controls
The salivary LDH levels in the HNSCC patients and normal subjects were estimated and compared. In the HNSCC group, LDH expression in the saliva taken from regions of the tongue and other areas of the oral cavity significantly increased over the levels observed in the saliva obtained from other areas (P<0.001). The expression of LDH in the saliva of other patients with HNSCC was similar to that in the saliva of the control group. The salivary LDH levels in the patients and the control group were compared with the LDH expression in tumor and healthy tissue margins (Table 1).
Discussion
For HNSCC patients, the survival rate is approximately five years after diagnosis a period that is not as long as that for many other cancers. This difference can be attributed to the failure to detect the disease early in its progression and the inadequate efficacy of therapeutic procedures (2–4). Early diagnosis and intervention for HNSCC is very important, thus highlighting the role of biomarkers in these processes. HNSCC biomarkers can be analyzed in tissue, plasma, and other bodily fluids, such as saliva. Salivary and serum biomarkers are essential tools for cancer diagnosis. The initial diagnosis of cancer can be based on the specific characteristics of cancer cells, which cause changes to serum or saliva; such changes can also be used as bases for initial diagnosis. If salivary changes are proportional to tumor progression, then saliva-based diagnosis can serve as a non-invasive method for the early detection of tumors (9, 12, 13, 23, 24).
The present study is the first to compare LDH levels in saliva and LDH expression in tissues.
According to this study, expression of the enzyme in affected patients was higher than that of normal people. As well, the expression level of this enzyme was higher in both saliva and tumor tissues compared with saliva of healthy individuals and the normal tissue margin of the tumor. Moreover, the expression level of this enzyme was reported higher in patients with SCC of the tongue and oral cavity compared to other parts of the HNSCC.
We observed increased LDH expression in the patients’ tumoral cells compared with the levels found in the healthy tissue margins. The comparison of LDH expression in the saliva of HNSCC patients and the matched healthy controls showed significantly increased LDH levels in the former.
Previous studies on salivary LDH expression in patients with oral squamous cell carcinoma (OSCC) and healthy individuals demonstrated that LDH expression was significantly higher in the patient group than in the control group, consistent with our results (25, 26). In their comparison of the total salivary LDH levels of three groups (25 healthy individuals, 25 individuals with oral leukoplakia, and 25 subjects with OSCC) Patel and Metgud found increasing levels from the healthy group to the OSCC group (27). In these studies, salivary LDH was evaluated only in OSCC patients, whereas in our research, such expression was evaluated in all patients with HNSCC. This approach allowed us to confirm the specificity of increasing LDH expression for a particular region. In the aforementioned studies, measurements of LDH expression in the saliva and tumoral and normal tissues of HNSCC patients were not performed simultaneously.
Some studies indicated that LDH expression in a tumor is significantly higher than that in normal tissue, and certain researchers found no significant increase in LDH expression and histopathological grade and stage. Other studies demonstrated that LDH levels increase in tumoral tissue proportionally with histopathological grade. Our results confirmed the first two findings but disagreed with the proportionality between LDH expression and histopathological grade and stage (28-32).
The early detection of cancer is important because it reduces mortality and morbidity. Given the invasive nature of most diagnostic methods, a crucial requirement is the development or identification of a non-invasive approach that can rapidly detect cancer with high accuracy, precision, and specificity. Through periodic measurements, salivary LDH in people at high risk of HNSCC can be used for screening and early detection. The other advantages of saliva-based testing over conventional methods are low cost and fast response.
Further research on this topic is required to elucidate the molecular mechanisms behind the metabolic variability of malignant tumors. Such efforts may advance the identification of new approaches to exploiting tumor glycolysis for treatment. If it is possible to compare LDH levels in the saliva of patients before and after HNSCC onset, it is also possible to measure the expression of this enzyme in the saliva of patients periodically at the time of initial diagnosis and during the course of treatment and disease progression. The results obtained would be more valuable, and the role of other influencing factors (excluding the disease itself) would be substantially reduced. We suggest that this non-invasive, fast, and inexpensive diagnostic method be used for other enzymes and substances that increase with respect to the functioning of cancer cells in serum and saliva.
This study is the first to compare salivary LDH expression in HNSCC patients and healthy sex- and age-matched individuals with the expression of the enzyme in the tumor and healthy tissue margins of the patients. Significantly increased LDH expression was found in the saliva of the patients compared with such expression in the healthy controls; the same increased expression was detected in the tumor tissues of the patients relative to that in the normal, healthy tissue margins. Because LDH expression increases simultaneously in tumor tissues and saliva, it can be a useful diagnostic indicator for the early detection and prediction of HNSCC.
Acknowledgements
This work was supported by the Mashhad University of Medical Sciences.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA, Trotti A, Pfister DG, Grandis JR. Head and neck cancer: recent advances and new standards of care. Journal of Clinical Oncology. 2006;24(17):2603–5. doi: 10.1200/JCO.2006.07.1464. [DOI] [PubMed] [Google Scholar]
- 3.HEROIU A-D, DANCIU CE, POPESCU CR. Multiple cancers of the head and neck. Maedica. 2013;8(1):80. [PMC free article] [PubMed] [Google Scholar]
- 4.Sanderson R, Ironside J. Squamous cell carcinomas of the head and neck. BMJ: British Medical Journal. 2002;325(7368):822. doi: 10.1136/bmj.325.7368.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abgral R, Querellou S, Potard G, Le Roux P-Y, Le Duc-Pennec A, Marianovski R, et al. Does 18F-FDG PET/CT improve the detection of posttreatment recurrence of head and neck squamous cell carcinoma in patients negative for disease on clinical follow-up? Journal of Nuclear Medicine. 2009;50(1):24–9. doi: 10.2967/jnumed.108.055806. [DOI] [PubMed] [Google Scholar]
- 6.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. Journal of clinical oncology. 2011;29(32):4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ragin C, Modugno F, Gollin S. The epidemiology and risk factors of head and neck cancer: a focus on human papillomavirus. Journal of dental research. 2007;86(2):104–14. doi: 10.1177/154405910708600202. [DOI] [PubMed] [Google Scholar]
- 8.Wierzbicka M, Szyfter K, Milecki P, Składowski K, Ramlau R. The rationale for HPV-related oropharyngeal cancer de-escalation treatment strategies. Contemporary Oncology. 2015;19(4):313. doi: 10.5114/wo.2015.54389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friemel J, Foraita R, Günther K, Heibeck M, Günther F, Pflueger M, et al. Pretreatment oral hygiene habits and survival of head and neck squamous cell carcinoma (HNSCC) patients. BMC oral health. 2016;16(1):33. doi: 10.1186/s12903-016-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamanaka RB, Chandel NS. Targeting glucose metabolism for cancer therapy. Journal of Experimental Medicine. 2012;209(2):211–5. doi: 10.1084/jem.20120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA: a cancer journal for clinicians. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 12.Lee K-D, Lee H-S, Jeon C-H. Body fluid biomarkers for early detection of head and neck squamous cell carcinomas. . Anticancer research. 2011;31(4):1161–7. [PubMed] [Google Scholar]
- 13.Miao P, Sheng S, Sun X, Liu J, Huang G. Lactate dehydrogenase A in cancer: a promising target for diagnosis and therapy. IUBMB life. 2013;65(11):904–10. doi: 10.1002/iub.1216. [DOI] [PubMed] [Google Scholar]
- 14.Shi M, Cui J, Du J, Wei D, Jia Z, Zhang J, et al. A novel KLF4/LDHA signaling pathway regulates aerobic glycolysis in and progression of pancreatic cancer. Clinical Cancer Research. 2014;20(16):4370–80. doi: 10.1158/1078-0432.CCR-14-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blatt S, Voelxen N, Sagheb K, Pabst AM, Walenta S, Schroeder T, et al. Lactate as a predictive marker for tumor recurrence in patients with head and neck squamous cell carcinoma (HNSCC) post radiation: a prospective study over 15 years. Clinical oral investigations. 2016;20(8):2097–104. doi: 10.1007/s00784-015-1699-6. [DOI] [PubMed] [Google Scholar]
- 16.Frezza C, Gottlieb E, editors. Mitochondria in cancer: not just innocent bystanders. Seminars in cancer biology. 2009 doi: 10.1016/j.semcancer.2008.11.008. Elsevier. [DOI] [PubMed] [Google Scholar]
- 17.Alfarouk KO, Shayoub ME, Muddathir AK, Elhassan GO, Bashir AH. Evolution of tumor metabolism might reflect carcinogenesis as a reverse evolution process (dismantling of multicellularity). . Cancers. 2011;3(3):3002–17. doi: 10.3390/cancers3033002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mydlarz WK, Hennessey PT, Califano JA. Advances and perspectives in the molecular diagnosis of head and neck cancer. Expert opinion on medical diagnostics. 2010;4(1):53–65. doi: 10.1517/17530050903338068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaaij-Visser TB, Brakenhoff RH, Leemans CR, Heck AJ, Slijper M. Protein biomarker discovery for head and neck cancer. Journal of proteomics. 2010;73(10):1790–803. doi: 10.1016/j.jprot.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Zhang HF, Wang YC, Han YD. MicroRNA34a inhibits liver cancer cell growth by reprogramming glucose metabolism. Molecular medicine reports. 2018 doi: 10.3892/mmr.2018.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryne M, Koppang HS, Lilleng R, Stene T, Bang G, Dabelsteen E. New malignancy grading is a better prognostic indicator than Broders' grading in oral squamous cell carcinomas. Journal of Oral Pathology & Medicine. 1989;18(8):432–7. doi: 10.1111/j.1600-0714.1989.tb01339.x. [DOI] [PubMed] [Google Scholar]
- 22.Garde-Noguera J, Gil-Raga M, Evgenyeva E, Bernet L, Llombart-Cussac A. Lactate Dehydrogenase-5 (Ldh-5) Immunohistochemical Expression as Predictor of Efficacy of First-Line Therapy in Patients with Advanced Colorectal Cancer Treated with Chemotherapy and Bevacizumab. J Clin Exp Pathol. 2016;6(293):2161–0681.1000293. [Google Scholar]
- 23.Bonakdaran S, Varasteh A-R. Correlation between serum 25 hydroxy vitamin D3 and laboratory risk markers of cardiovascular diseases in type 2 diabetic patients. Saudi Med J. 2009;30(4):509–14. [PubMed] [Google Scholar]
- 24.Hua S, Liu C, Liu L, Wu D. miR-142-3p inhibits aerobic glycolysis and cell proliferation in hepatocellular carcinoma via targeting LDHA. Biochemical and biophysical research communications. 2018 doi: 10.1016/j.bbrc.2018.01.112. [DOI] [PubMed] [Google Scholar]
- 25.Lokesh K, Kannabiran J, Rao MD. Salivary Lactate Dehydrogenase (LDH)-A Novel Technique in Oral Cancer Detection and Diagnosis. Journal of clinical and diagnostic research: JCDR. 2016;10(2):ZC34. doi: 10.7860/JCDR/2016/16243.7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shpitzer T, Bahar G, Feinmesser R, Nagler RM. A comprehensive salivary analysis for oral cancer diagnosis. Journal of cancer research and clinical oncology. 2007;133(9):613–7. doi: 10.1007/s00432-007-0207-z. [DOI] [PubMed] [Google Scholar]
- 27.Patel S, Metgud R. Estimation of salivary lactate dehydrogenase in oral leukoplakia and oral squamous cell carcinoma: a biochemical study. Journal of cancer research and therapeutics. 2015;11(1):119. doi: 10.4103/0973-1482.138193. [DOI] [PubMed] [Google Scholar]
- 28.Dong T, Liu Z, Xuan Q, Wang Z, Ma W, Zhang Q. Tumor LDH-A expression and serum LDH status are two metabolic predictors for triple negative breast cancer brain metastasis. Scientific reports. 2017;7(1):6069. doi: 10.1038/s41598-017-06378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koukourakis M, Giatromanolaki A, Sivridis E, Bougioukas G, Didilis V, Gatter K. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. British journal of cancer. 2003;89(5):877–85. doi: 10.1038/sj.bjc.6601205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Augoff K, Grabowski K. Significance of lactate dehydrogenase measurements in diagnosis of malignancies. . Polski merkuriusz lekarski: organ Polskiego Towarzystwa Lekarskiego. 2004;17(102):644–7. [PubMed] [Google Scholar]
- 31.Arora R, Schmitt D, Karanam B, Tan M, Yates C, Dean-Colomb W. Inhibition of the Warburg effect with a natural compound reveals a novel measurement for determining the metastatic potential of breast cancers. Oncotarget. 2015;6(2):662. doi: 10.18632/oncotarget.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolev Y, Uetake H, Takagi Y, Sugihara K. Lactate dehydrogenase-5 (LDH-5) expression in human gastric cancer: association with hypoxia-inducible factor (HIF-1α) pathway, angiogenic factors production and poor prognosis. Annals of surgical oncology. 2008;15(8):2336–44. doi: 10.1245/s10434-008-9955-5. [DOI] [PubMed] [Google Scholar]


