Abstract
Trauma-associated sleep disorder (TASD) is a parasomnia sharing characteristics of post-traumatic stress disorder (PTSD) and REM sleep behavior disorder (RBD) including dream-enactment behavior (DEB). Here we report REM sleep without atonia (RSWA) and other neurological features in a patient with complex vocal and motor DEB following traumatic combat military exposure. Post-discharge, his wife observed frequent yelling and jerking during sleep with dream mentation reminiscent of traumatic military experiences. He was initially diagnosed with PTSD. Polysomnography demonstrated RSWA and severe obstructive sleep apnea treated with nasal continuous positive airway pressure (CPAP). Dream-enactment behavior severity and frequency was reduced, but still persisted despite nasal CPAP and sequential fluoxetine, escitalopram, prazosin, and melatonin trials. Our case demonstrated overlapping clinical features of PTSD and RBD with polysomnography features of RSWA supportive of idiopathic RBD but no “soft signs” suggesting underlying synucleinopathy. Longitudinal follow-up of larger case series must clarify whether TASD consistently manifests REM sleep atonia loss and determine the phenoconversion risk for synucleinopathy neurodegeneration.
Commentary:
A commentary on this article appears in this issue on page 181.
Citation:
Feemster JC, Smith KL, McCarter SJ, St. Louis EK. Trauma-associated sleep disorder: a posttraumatic stress/REM sleep behavior disorder mash-up? J Clin Sleep Med. 2019;15(2):345–349.
Keywords: posttraumatic stress disorder, trauma associated sleep disorder, REM sleep behavior disorder, polysomnography
INTRODUCTION
Trauma survivors with and without posttraumatic stress disorder (PTSD) frequently report a variety of sleep disturbances, consisting of trauma-related nightmares, autonomic hyper-arousal, and excessive movements.1 Sleep disturbances following a traumatic experience are predictors for physical and psychiatric symptoms, and there appear to be bidirectional relationships between sleep, mood, anxiety, and PTSD.2–4 Trauma-associated sleep disorder (TASD) is a proposed parasomnia distinct from nightmare disorder (NMD), non-rapid eye movement (NREM) sleep disorders of arousal, and rapid eye movement (REM) sleep behavior disorder (RBD), although TASD has some overlapping clinical features with each of these entities.1,5,6 The most notable distinction between patients with NMD and TASD is the occurrence of dream enactment behavior (DEB) with excessive movements and complex vocal and motor behaviors during sleep, similar to those seen in RBD.6 Patients with TASD have been described to have variable features of excessive muscle activity in both NREM and REM sleep. Loss or dysregulation of normal REM sleep atonia in TASD appears similar to that seen in RBD. In TASD, however, a distinctive patient history of an acute onset of DEB and nightmares occurs in close temporal proximity to trauma and other features that are unusual or inconsistent with RBD, such as a nightmare theme that is specifically linked to the personally-experienced traumatic event or exposure that is similar to the “flashbacks” seen in PTSD, and TASD often presents at a considerably younger age than is typical for RBD.6 We present a case of probable TASD, describe other detailed neurological and polysomnography (PSG) features, and discuss implications for the understanding of TASD and further steps needed to delineate its standing as a possible unique parasomnia distinct from RBD and NMD, and discuss considerations about whether TASD may be a separable entity from PTSD.
REPORT OF CASE
A 53-year-old man presented with DEB and complex motor behavior during sleep. He had been previously diagnosed with PTSD following military service as a United States Army mechanic in the Iraqi theater from 2005 to 2006, although he did not seem to have prominent daytime symptoms of hyper-vigilance, irritability, or aggression or daytime episodes involving re-experience of traumatic exposures consistent with flashbacks. Shortly following his return from service in Iraq, his wife noted that he exhibited violent sleep movements, yelling, or jerking in his sleep, about once or twice per night, that had not been previously present before his tour of duty. Behaviors reportedly directly paralleled dream mentation concerning his military exposure including vocalizations in which he would yell “get down!” or that there are “incoming” (shells/ explosions). The themes of his nightmares involved his typical military work, which had involved dismantling cars hit by improvised explosive devices, gathering personal belongings of soldiers who had been killed, and witnessing several young soldiers sent into the field who never returned. A therapeutic trial of prazosin 4 mg daily was ineffective. He also evolved depressive symptoms and received antidepressant therapy with escitalopram 10 mg, later changed to fluoxetine 20 mg, and following this therapeutic change in 2016, his DEB frequency and severity was reduced, although it continued to occur with about one dream enactment episode occurring every 4 to 6 weeks. At the time of his initial visit in the sleep clinic in 2017, he was continuing to receive fluoxetine 20 mg. His only other medications included losartan and amlodipine for high blood pressure. There was no current or previous remote childhood history of sleep walking, falling from the bed, injury to himself or bed partner, nor symptoms of hyposmia, constipation, or memory loss. The patient also noted a history of snoring, occasionally disturbing in volume to his wife, and she had reported seeing him stop breathing during sleep. His Epworth Sleepiness Scale score was 4. He maintained a regular sleep schedule from about 11:00 pm to 7:00 am, with mild initial insomnia and an approximate sleep onset time of 60 minutes, then sleeping soundly despite traumatic nightmares and DEB.
Physical and neurological examination, including a detailed motor examination for motor signs of parkinsonism were normal, without rigidity, bradykinesia, tremor, or postural instability. After informed consent, additional research measures were also performed to investigate possible signs of underlying synucleinopathy, and on each of these measures he demonstrated normal performance, including the Montreal Cognitive Assessment (MoCa score 28), Kokmen Short Test of Mental Status (score 36), brief smell identification test (B-SIT score 11), King-Devick Test (KDT score 12.9 seconds), D15 color-vision test, Rolyan grooved pegboard test (dominant/non-dominant times 17.8/16.8 seconds), timed up-and-go test (7.5 seconds), and 10-meter walk (4.2 seconds).7–15
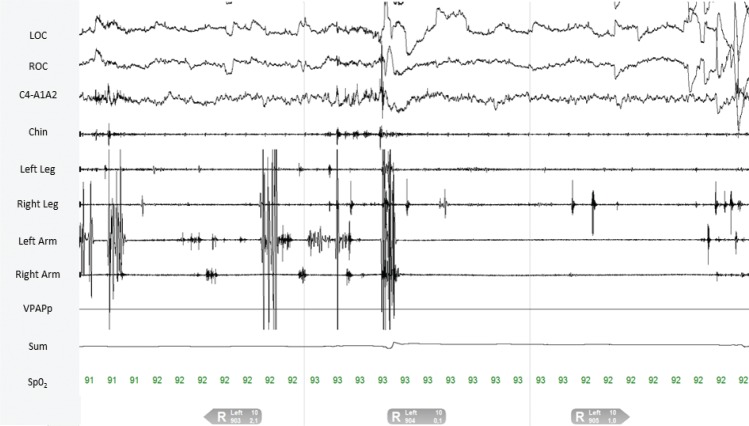
PSG demonstrated an apnea-hypopnea index of 21 events/h during the diagnostic study. A therapeutic trial of nasal continuous positive airway pressure (CPAP) was effective in eliminating sleep-disordered breathing events and snoring at 9 cm H2O. Quantitative analysis of REM sleep muscle activity by our previously established methods demonstrated abnormal levels of REM sleep without atonia (RSWA), yet in a distribution somewhat atypical for synucleinopathy-associated RBD cases and instead similar to that often seen in patients receiving antidepressant medications, with more prominent RSWA in the anterior tibialis muscles (Table 1 and Figure 1).16–18 The right leg phasic density, automated Ferri REM atonia index, average chin duration, and left-leg phasic-burst duration all exceeded RBD diagnostic threshold values, but muscle activity remained within normal limits for visual/manual analyses in the submentalis and flexor digitorum superficialis muscles which are often considered most specific for RBD diagnosis.18 Video review of selected epochs demonstrated excessive myoclonic jerking, with both axial body jerks and jerks of the limbs during both the diagnostic study and CPAP treatment trial. However, there were no complex oneiric vocal or motor behaviors captured during PSG. A therapeutic trial of melatonin was recommended, beginning with 3 mg, incrementing to 6 mg if the lower dose was ineffective, and nasal CPAP was prescribed.
Table 1.
Quantitative RSWA analysis in a 53-year-old man with probable trauma-associated sleep disorder.
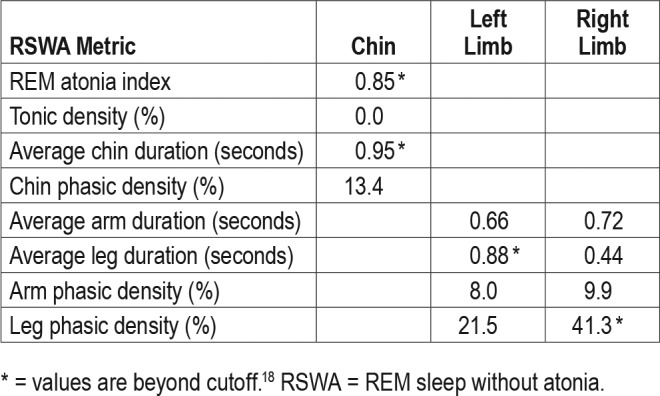
Figure 1. 30-second epoch demonstrating RSWA in a 53-year-old man with probable trauma-associated sleep disorder.
RSWA = REM sleep without atonia.
At 15 months clinical follow-up, the patient was faithfully utilizing nasal CPAP, reporting that it helped him feel more rested. However, he continued to experience episodes of traumatic nightmares and complex motor DEB including screaming, shouting, vocalizing, punching, and kicking behaviors one night per month. He reported that he had discontinued melatonin after the first two months, finding it to have been ineffective in altering dream enactment behavior frequency or severity.
DISCUSSION
The proposed diagnostic criteria for trauma-associated sleep disorder include:1,6
Inciting traumatic experience.
A history of altered dream mentation that is related to prior traumatic experience.
Self or witnessed reports of DEB including abnormal vocalizations or abnormal motor behaviors in sleep.
Symptoms of autonomic hyperarousal or monitoring that demonstrate tachycardia, tachypnea, or diaphoresis not due to sleep-disordered breathing.
A PSG demonstrating RSWA or DEB in REM sleep.
Absence of electroencephalographic epileptiform activity on PSG.
Our patient fulfilled most of the proposed diagnostic criteria for TASD, lacking only clear signs of autonomic hyperarousal. Following the inciting traumatic military experience, DEB was observed by his wife and reportedly involved nightmares that directly paralleled his traumatic military experience, and he had features of RSWA, albeit in a somewhat atypical distribution from “traditional” idiopathic/isolated or symptomatic RBD, which is usually presumed to be caused by synucleinopathy. In this case, the strong temporal relationship between a history of directly preceding inciting traumatic experience in the military prior to onset of DEB, the specificity of traumatic dream content and themes linked to the patient's own individual traumatic military exposures, and DEB-mirroring dream content of a traumatic nature all support the diagnosis of the proposed parasomnia TASD. Some might argue that the case is simply a PTSD case, and indeed, our case had been previously diagnosed with PTSD by a psychiatrist. The diagnosis of PTSD by DSM-5 criteria requires the following elements: direct exposure to a significantly traumatic event involving actual or threatened death or injury; persistent re-experiencing of the event including nightmares and/or flashbacks; avoidance of trauma-related stimuli; negative thoughts or feelings beginning or worsening after the traumatic exposure; trauma-related arousal and reactivity including irritability, aggression, hyper-vigilance, and difficulty with concentration and/or sleeping; symptoms causing significant distress or impairment; and a duration of at least one month.19 However, our case had significantly greater complex dream enacting motor behaviors that are atypical for a PTSD diagnosis alone. Other features that argue for the coexistence of a parasomnia disorder include the complex motor and vocal dream-enactment behaviors accompanying his dreams, the prominence of the REM sleep atonia loss (RSWA) which excludes “pseudo-RBD” (ie, complex motor behaviors associated with sleep apnea arousal that mimic RBD),20 and the absence of features of hypervigilance or re-experiencing of the event (“flashbacks”) in the daytime. Additionally, to further differentiate this patient from “pseudo-RBD,” abnormal sleep behaviors occurred spontaneously, not during apnea-induced arousals, and did not improve during administration of continuous positive airway pressure therapy.20 However, the alternative diagnosis of idiopathic/isolated RBD could also be very reasonably applied to this patient and is, in fact, the correct diagnosis according to current ICSD-3 criteria and nomenclature.
A potential common pathophysiology linking PTSD with RBD has been suggested by overlap of clinical phenomena and by evidence from neurophysiologic, neuroimaging, and neuropathological studies.21,22 Sleep disturbance is common among patients with PTSD. One study found decreased slow-wave sleep in patients with PTSD.23 Additionally, discrepancies between self-reported and objectively-measured sleep parameters have also been associated with trauma exposure or PTSD, challenging prior assertions that individuals with PTSD over-report their sleep disturbances.24 One study found Vietnam combat veterans with PTSD had a higher percentage of REM-sleep epochs with at least one prolonged twitch burst, although RSWA was not formally analyzed.25 Severe anxiety disorders such as PTSD have shown altered cortical thickness and regional brain volumes and post-mortem neuronal counts of structures involved in or closely related to REM sleep control and dreaming.26–31 Alterations in amygdala and hippocampal volumes have been found in PTSD, with consistent findings of smaller hippocampi and either increased or decreased amygdalar volumes compared with controls.27,30,31 In particular, right amygdala volume loss has been associated with anxious arousal symptomatology in PTSD.32 Several human and animal studies have suggested altered noradrenergic functioning in the locus ceruleus (LC) in patients with PTSD, with evidence pointing to stress-induced LC neuronal loss and altered compensatory function in the LC region causing behavioral symptoms of anxious arousal and hypervigilance.32–34 One theory of the bidirectional relationship between sleep and PTSD holds that patients with PTSD have enhanced REM-on and wake-promoting amygdala and medial prefrontal cortex network activity, with corresponding decreased REM-off and anterior hypothalamic sleep-facilitating network activity.35
Patients with PTSD have decreased neuronal counts in the LC and peri-locus ceruleus nuclei located in the dorsal pontine tegmentum, corresponding closely to a directly neighboring region where the REM-onset neurons of the subceruleus/sub-lateral dorsal nucleus are located, which also regulates REM sleep atonia.21,22,36 Decreased LC regional neurons could result in decreased neuronal output of the LC and neighboring structures to other REM sleep-modulating nuclei such as the pedunculopontine and magnocellularis nuclei, subsequently altering the output of these structures,22 similar to the pathophysio-logic process underlying RBD and accounting for both loss of normal REM sleep atonia regulation and increased motor activity during REM sleep as seen in RBD.37
Further research will be necessary to determine if TASD is a distinct disorder that evolves from PTSD or if it is, instead, a special interaction between PTSD and RBD, possibly caused by an interaction between PTSD symptoms in an individual harboring covert preexisting synuclein pathology in the dorsal pons that would otherwise be asymptomatic. In the latter possibility of interaction between PTSD and RBD, pathophysiology of PTSD in the dorsal pons and traumatic nightmares could facilitate expression of enactment of PTSD dreams. This is a similar scenario to theories in which antidepressant medications are associated with RBD. The development of antidepressant-associated RBD has been proposed as an early signal of an underlying covert neurodegenerative disease, since patients who are either receiving or not receiving antidepressants show a similar frequency of neurodegenerative biomarkers. However, patients receiving antidepressants phenoconverted to overt neurodegenerative disorders at a lower frequency than those who did not receive antidepressants.38 This study suggested that antidepressant medications act to promote and unveil RBD symptoms at an earlier timeframe and, through lead-time bias, lead to earlier detection of RBD in the setting of antidepressant use, in comparison to patients with RBD who do not receive antidepressant medications.38 Others have interpreted these data differently, pointing out that a neuroprotective effect of the antidepressants might delay the progression of underlying neurodegenerative disease.39 Alternatively, anti-depressants may have a more directly causal role in RBD, possibly producing a functional, reversible disturbance of REM sleep atonia regulation.40
A proposed treatment for TASD is prazosin, a centrally active alpha-1-adrenergic receptor antagonist, which is also a standard treatment for PTSD-associated trauma nightmares.41 Several clinical trials, including a meta-analysis of placebo-controlled studies on PTSD sleep disturbances, found prazosin significantly improves nightmare frequency, PTSD severity, and sleep quality. These findings support the efficacy of this medication for the treatment of combat veterans and trauma survivors.42–44 Due to improvement in symptoms following treatment with prazosin, TASD could be driven by hyper-adrenergic function, which may aid diagnostic distinction from RBD, since prazosin would not be expected to be effective in RBD (although systematic treatment trials are currently lacking in either TASD or RBD). On the other hand, a recent large multi-center trial of prazosin for alleviating distressing dreams or improving sleep quality of military veterans with chronic PTSD showed no beneficial outcomes for prazosin.45 In our case, prazosin 4 mg prior to bedtime was similarly not beneficial in reducing DEB frequency or severity, although it remains unclear if our case was an unusual variant of PTSD, or more likely was the distinctive parasomnia TASD for which prazosin is as yet unproven. It has been suggested that prazosin may not be effective in patients with chronic PTSD with obstructive sleep apnea due to interference of the drug's mechanism or masking of its beneficial effects.45 In our patient, melatonin was also ineffective, at least when dosed to the average effective dose of 6 mg nightly.46 Further controlled studies of prazosin and other treatments typically effective for RBD (melatonin, clonazepam) will need to be done to clarify which treatment strategies are safe and effective for the management of TASD.
The fundamental mechanisms of TASD and its overlapping symptoms with other parasomnias and PTSD are complex and poorly understood. TASD may be part of the spectrum of idiopathic RBD that onsets before age 60, in relationship to mood, anxiety, or PTSD, with or without antidepressant treatment. Such cases might be considered distinct from “traditional” idiopathic/isolated RBD in older adults, when RBD is presumably associated with underlying synucleinopathy in most cases. Such younger age-onset RBD cases associated with antidepressants have been described as “psychiatric RBD,” an interesting, possibly distinct subset of patients who develop RBD symptoms at a younger age and who may have a different ultimate prognosis (or at least, a considerably longer time course) for the risk of developing overt neurodegenerative disease. Further prospective and longitudinal follow-up of this and other cases will be necessary to determine whether TASD is a distinct parasomnia or simply a variant “mash-up” of PTSD and RBD and to determine phenoconversion risk in this interesting subset of patients presenting with dream enactment.
DISCLOSURE STATEMENT
The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 1 UL1 RR024150-01, and by the Mayo Clinic Alzheimer's Disease Research Center (ADRC), through Grant Number P50 AG016574. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. All authors have seen and approved the final version of this manuscript. Messrs. Feemster and Smith and Dr. McCarter report no disclosures. Dr. St Louis receives research support from the Mayo Clinic Center for Translational Science Activities (CCaTS), supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 1 UL1 RR024150-01; from the Mayo Clinic Alzheimer's Disease Research Center Grant Award from the National Institute on Aging (P50 AG016574); from Michael J. Fox Foundation; and from Sunovion, inc. He has also served as a consultant for Axovant, Inc., but receives no personal fees.
ACKNOWLEDGMENTS
The authors are grateful for secretarial support in manuscript preparation and submission from Ms. Lea Dacy, Mayo Clinic Department of Neurology.
REFERENCES
- 1.Mysliwiec V, O'Reilly B, Polchinski J, Kwon HP, Germain A, Roth BJ. Trauma associated sleep disorder: a proposed parasomnia encompassing disruptive nocturnal behaviors, nightmares, and REM without atonia in trauma survivors. J Clin Sleep Med. 2014;10(10):1143–1148. doi: 10.5664/jcsm.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavie P. Sleep disturbances in the wake of traumatic events. N Engl J Med. 2001;345(25):1825–1832. doi: 10.1056/NEJMra012893. [DOI] [PubMed] [Google Scholar]
- 3.Wellman LL, Fitzpatrick ME, Hallum OY, Sutton AM, Williams BL, Sanford LD. Individual differences in animal stress models: considering resilience, vulnerability, and the amygdala in mediating the effects of stress and conditioned fear on sleep. Sleep. 2016;39(6):1293–1303. doi: 10.5665/sleep.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werner KB, Griffin MG, Galovski TE. Objective and subjective measurement of sleep disturbance in female trauma survivors with posttraumatic stress disorder. Psychiatry Res. 2016;240:234–240. doi: 10.1016/j.psychres.2016.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mellman TA, Pigeon WR, Nowell PD, Nolan B. Relationships between REM sleep findings and PTSD symptoms during the early aftermath of trauma. J Trauma Stress. 2007;20(5):893–901. doi: 10.1002/jts.20246. [DOI] [PubMed] [Google Scholar]
- 6.Mysliwiec V, Brock MS, Creamer JL, O'Reilly BM, Germain A, Roth BJ. Trauma associated sleep disorder: a parasomnia induced by trauma. Sleep Med Rev. 2018;37:94–104. doi: 10.1016/j.smrv.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Freitas S, Simões MR, Alves L, Santana I. Montreal cognitive assessment: validation study for mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord. 2013;27(1):37–43. doi: 10.1097/WAD.0b013e3182420bfe. [DOI] [PubMed] [Google Scholar]
- 8.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 9.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol. 1991;48(7):725–728. doi: 10.1001/archneur.1991.00530190071018. [DOI] [PubMed] [Google Scholar]
- 10.Menon C, Westervelt HJ, Jahn DR, Dressel JA, O'Bryant SE. Normative performance on the Brief Smell Identification Test (BSIT) in a multi-ethnic bilingual cohort: a Project FRONTIER study. Clin Neuropsychol. 2013;27(6):946–961. doi: 10.1080/13854046.2013.796406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galetta KM, Chapman KR, Essis MD, et al. Screening utility of the King-Devick Test in mild cognitive impairment and Alzheimer disease dementia. Alzheimer Dis Assoc Disord. 2017;31(2):152–158. doi: 10.1097/WAD.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneck ME, Haegerstrom-Portnoy G, Lott LA, Brabyn JA. Comparison of panel D-15 tests in a large older population. Optom Vis Sci. 2014;91(3):284–290. doi: 10.1097/OPX.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desrosiers J, Hebert R, Bravo G, Dutil E. The Purdue Pegboard Test: normative data for people aged 60 and over. Disabil Rehabil. 1995;17(5):217–224. doi: 10.3109/09638289509166638. [DOI] [PubMed] [Google Scholar]
- 14.Bohannon RW. Reference values for the timed up and go test: a descriptive meta-analysis. J Geriatr Phys Ther. 2006;29(2):64–68. doi: 10.1519/00139143-200608000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Bohannon RW. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. 1997;26(1):15–19. doi: 10.1093/ageing/26.1.15. [DOI] [PubMed] [Google Scholar]
- 16.McCarter SJ, St Louis EK, Duwell EJ, et al. Diagnostic thresholds for quantitative REM sleep phasic burst duration, phasic and tonic muscle activity, and REM atonia index in REM sleep behavior disorder with and without comorbid obstructive sleep apnea. Sleep. 2014;37(10):1649–1662. doi: 10.5665/sleep.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarter SJ, St Louis EK, Sandness DJ, et al. Antidepressants increase REM sleep muscle tone in patients with and without REM sleep behavior disorder. Sleep. 2015;38(6):907–917. doi: 10.5665/sleep.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarter SJ, St Louis EK, Sandness DJ, et al. Diagnostic REM sleep muscle activity thresholds in patients with idiopathic REM sleep behavior disorder with and without obstructive sleep apnea. Sleep Med. 2017;33:23–29. doi: 10.1016/j.sleep.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 20.Iranzo A, Santamaria J. Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep. 2005;28(2):203–206. doi: 10.1093/sleep/28.2.203. [DOI] [PubMed] [Google Scholar]
- 21.Freeman T, Karson C, Garcia-Rill E. Locus coeruleus neuropathology in anxiety disorders [abstract] Biol Psychiatry. 1993;33(6):A148. [Google Scholar]
- 22.Garcia-Rill E. Disorders of the reticular activating system. Med Hypotheses. 1997;49(5):379–387. doi: 10.1016/s0306-9877(97)90083-9. [DOI] [PubMed] [Google Scholar]
- 23.Yetkin S, Aydin H, Ozgen F. Polysomnography in patients with post-traumatic stress disorder. Psychiatry Clin Neurosci. 2010;64(3):309–317. doi: 10.1111/j.1440-1819.2010.02084.x. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi I, Huntley E, Lavela J, Mellman TA. Subjectively and objectively measured sleep with and without posttraumatic stress disorder and trauma exposure. Sleep. 2012;35(7):957–965. doi: 10.5665/sleep.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross RJ, Ball WA, Dinges DF, et al. Motor dysfunction during sleep in posttraumatic stress disorder. Sleep. 1994;17(8):723–732. doi: 10.1093/sleep/17.8.723. [DOI] [PubMed] [Google Scholar]
- 26.Averill LA, Abdallah CG, Pietrzak RH, et al. Combat exposure severity is associated with reduced cortical thickness in combat veterans: a preliminary report. Chronic Stress (Thousand Oaks) 2017;1:1–9. doi: 10.1177/2470547017724714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodward SH, Kaloupek DG, Streeter CC, et al. Hippocampal volume, PTSD, and alcoholism in combat veterans. Am J Psychiatry. 2006;163(4):674–681. doi: 10.1176/ajp.2006.163.4.674. [DOI] [PubMed] [Google Scholar]
- 28.Hedges DW, Thatcher GW, Bennett PJ, et al. Brain integrity and cerebral atrophy in Vietnam combat veterans with and without posttraumatic stress disorder. Neurocase. 2007;13(5):402–410. doi: 10.1080/13554790701851551. [DOI] [PubMed] [Google Scholar]
- 29.Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30(7):1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Kuo JR, Kaloupek DG, Woodward SH. Amygdala volume in combat-exposed veterans with and without posttraumatic stress disorder: a cross-sectional study. Arch Gen Psychiatry. 2012;69(10):1080–1086. doi: 10.1001/archgenpsychiatry.2012.73. [DOI] [PubMed] [Google Scholar]
- 31.Morey RA, Gold AL, LaBar KS, et al. Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Arch Gen Psychiatry. 2012;69(11):1169–1178. doi: 10.1001/archgenpsychiatry.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naegeli C, Zeffiro T, Piccirelli M, et al. Locus coeruleus activity mediates hyperresponsiveness in posttraumatic stress disorder. Biol Psychiatry. 2018;83(3):254–262. doi: 10.1016/j.biopsych.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 33.Vanderheyden WM, Poe GR, Liberzon I. Trauma exposure and sleep: using a rodent model to understand sleep function in PTSD. Exp Brain Res. 2014;232(5):1575–1584. doi: 10.1007/s00221-014-3890-4. [DOI] [PubMed] [Google Scholar]
- 34.Pietrzak RH, Gallezot JD, Ding YS, et al. Association of posttraumatic stress disorder with reduced in vivo norepinephrine transporter availability in the locus coeruleus. JAMA Psychiatry. 2013;70(11):1199–1205. doi: 10.1001/jamapsychiatry.2013.399. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Germain A, Buysse DJ, Nofzinger E. Sleep-specific mechanisms underlying posttraumatic stress disorder: integrative review and neurobiological hypotheses. Sleep Med Rev. 2008;12(3):185–195. doi: 10.1016/j.smrv.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bracha HS, Garcia-Rill E, Mrak RE, Skinner R. Postmortem locus coeruleus neuron count in three American veterans with probable or possible war-related PTSD. J Neuropsychiatry Clin Neurosci. 2005;17(4):503–509. doi: 10.1176/appi.neuropsych.17.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Husain AM, Miller PP, Carwile ST. REM sleep behavior disorder: potential relationship to post-traumatic stress disorder. J Clin Neurophysiol. 2001;18(2):148–157. doi: 10.1097/00004691-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Postuma RB, Gagnon JF, Tuineaig M, et al. Antidepressants and REM sleep behavior disorder: isolated side effect or neurodegenerative signal? Sleep. 2013;36(11):1579–1585. doi: 10.5665/sleep.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolla BP, Mansukhani MP. Antidepressants trigger an early clinical presentation of REM sleep behavior disorder: the jury is still out. Sleep. 2014;37(8):1393. doi: 10.5665/sleep.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ju YE, Larson-Prior L, Duntley S. Changing demographics in REM sleep behavior disorder: possible effect of autoimmunity and antidepressants. Sleep Med. 2011;12(3):278–283. doi: 10.1016/j.sleep.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 41.Raskind MA, Dobie DJ, Kanter ED, Petrie EC, Thompson CE, Peskind ER. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J Clin Psychiatry. 2000;61(2):129–133. doi: 10.4088/jcp.v61n0208. [DOI] [PubMed] [Google Scholar]
- 42.Raskind MA, Thompson C, Petrie EC, et al. Prazosin reduces nightmares in combat veterans with posttraumatic stress disorder. J Clin Psychiatry. 2002;63(7):565–568. doi: 10.4088/jcp.v63n0705. [DOI] [PubMed] [Google Scholar]
- 43.Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- 44.Miller KE, Brownlow JA, Woodward S, Gehrman PR. Sleep and dreaming in posttraumatic stress disorder. Curr Psychiatry Rep. 2017;19(10):71. doi: 10.1007/s11920-017-0827-1. [DOI] [PubMed] [Google Scholar]
- 45.Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507–517. doi: 10.1056/NEJMoa1507598. [DOI] [PubMed] [Google Scholar]
- 46.McCarter SJ, Boswell CL, St. Louis EK, et al. Treatment outcomes in REM sleep behavior disorder. Sleep Med. 2013;14(3):237–242. doi: 10.1016/j.sleep.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]