Fig. 3. The Penn strain binds to sialylated glycans and to glycan fractions containing phosphorylated structures.
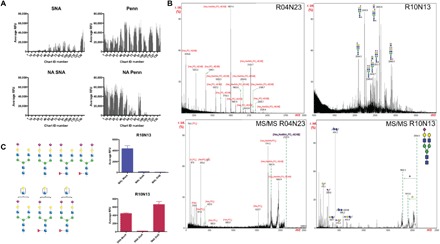
(A) NA treatment of HL-SGM and interrogation with SNA (25 μg/ml; detected with cyanine 5–conjugated streptavidin) and Penn reveal that IAV binds to array after removal of Sia as determined by loss of SNA binding. (B) Matrix-assisted laser desorption/ionization–time-of-flight MS (MALDI-TOF-MS) analysis of fractions, R10N13 (chart ID 118; high binding) indicating sialylated N-glycans and R04N23 (chart ID 47; high binding after NA treatment) indicating phosphorylated glycans, and tandem MS (MS/MS) of selected peaks. (C) Neuraminidase treatment of highest binding fraction R10N13 (predicted structures shown to the left; blue square, N-acetylglucosamine; red triangle, fucose; green circle, mannose; yellow circle, galactose; purple diamond, Sia) and binding of SNA (red bars) and MAL-I (blue bars) reveal the presence of both linkage types within the fraction.
