Fig. 4.
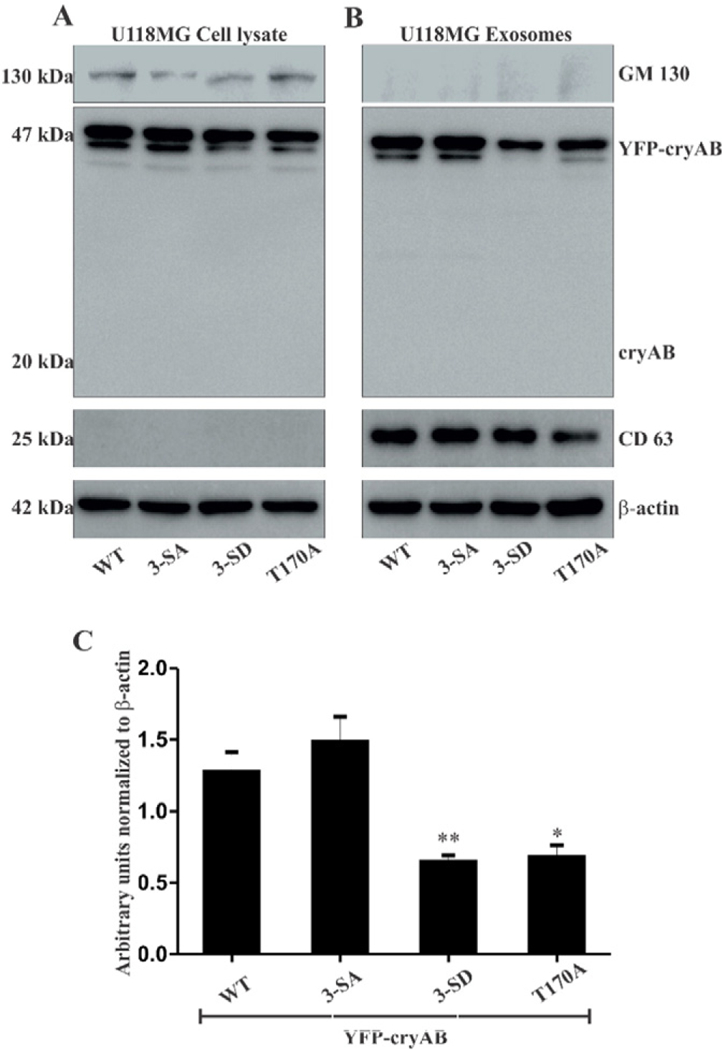
(A) WB of U118MG cells transiently transfected with WT, 3-SA, 3-SD and T170A YFP-cryAB constructs. (B) WB of exosomes isolated from culture media of transfected U118MG cells. YFP-cryAB constructs expressed in U118MG cells were secreted via exosomes, WT and 3-SA appeared to be secreted in fairly large quantities while levels of 3-SD secreted were reduced. Lack of a cryAB band at 20 kDa mark suggests that the YFP tag in YFP-cryAB was not cleaved off. GM130, a Golgi marker protein, was detected in the (A) U118MG cells, but (B) absent in the exosome lanes. Exosome marker protein, (B) CD63 was found enriched in the exosome lanes. β-Actin was used as a loading control. (C) Quantification of the Western blots showed a significant decrease in levels of phosphomimic (3-SD) and non-O-GlcNAcylatable (T170A) YFP-cryAB in isolated exosomes. β-Actin was used as loading control. Statistical significance determined by one way ANOVA, values indicate mean ± SEM (n = 3), (* = p < 0.05, ** = p < 0.01.
