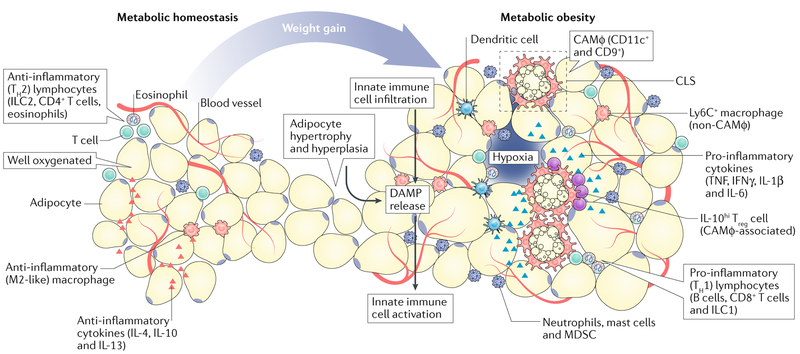
Fig. 2 |. Evolution of the adipose tissue microenvironment during obesity.
During healthy body-weight conditions (metabolic homeostasis), the adipose tissue microenvironment (ATME) is well-vascularized and rich in anti-inflammatory cytokines (such as IL-4, IL-10 and IL-13), and as a consequence, hosts a variety of type 2 immune cells, including alternatively activated (M2-like) macrophages, group 2 innate lymphoid cells, type 2 T helper (TH2) cells and IL-4-producing eosinophils. In response to body-weight gain or metabolic obesity, adipocytes undergo hyperplasia and hypertrophy; as the vascular supply becomes limited, these cells become stressed or die. This releases damage-associated molecular patterns (DAMP) into the microenvironment, which trigger the infiltration and activation of innate immunecells (for example, dendritic cells, macrophages and granulocytes). These effects promote the development of crown-like structures (CLS) and type 1 (pro-inflammatory) immune responses. This response includes accumulation of type 1 cytokines (for example TNF, IFNγ, IL-1β and IL-6), and pro-inflammatory immune cells, including various granulocytes, group 1 innate lymphoid cells, B cells and CD8+T cells, which perpetuate chronic inflammatory responses. Macrophages are highly diverse within the obese ATME; those associated with CLS (CLS-associated macrophage, CAMφ) proliferate and express the cell surface markers CD11c and CD9, while those that are further away (non-CAMφ) express lymphocyte antigen 6C (Ly6C). These inflammatory changes coincide with chronic fibrosis and vascular inflammation, which feed-forward to sustain inflammation. IL-10hi Treg cells, T regulatory cells producing high levels of IL-10; ILC1, group 1 innate lymphoid cells; MDSC, myeloid-derived suppressor cells.