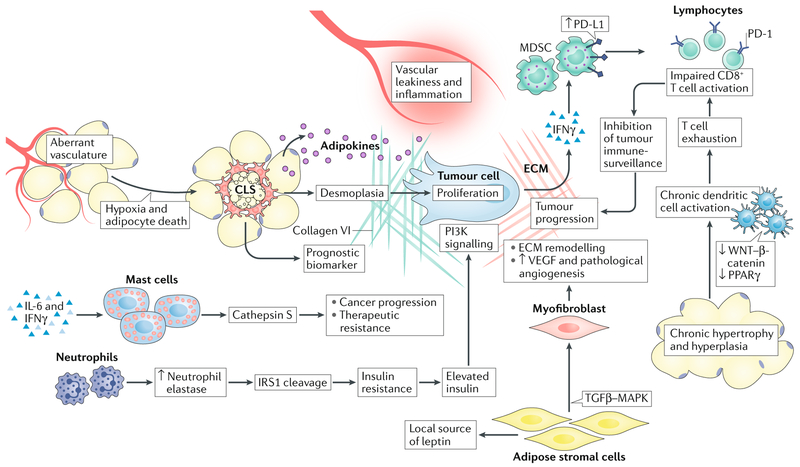
Fig. 3 |. Interactions between tumour cells and cells within the obese adipose tissue microenvironment.
Tumour cell biology is affected by multiple cellular players in the adipose tissue microenvironment (ATME). The enzyme neutrophil elastase cleaves insulin receptor substrate 1 (IRS1), leading to insulin resistance and elevated levels of free insulin, which can enhance phosphoinositide 3-kinase (PI3K) signalling within tumour cells. Mast cells produce various proteases such as cathepsin S, which can promote cancer progression and therapeutic resistance to cytotoxic therapies. Adipose stromal cells and myofibroblasts promote extracellular matrix (ECM) remodelling and facilitate tumour angiogenesis through vascular endothelial growth factor (VEGF) production. However, angiogenic vessels are dysfunctional, and when combined with adipocyte hypertrophy this triggers innate immune responses by macrophages and dendritic cells. Macrophages form crown-like structures (CLS) that not only serve as biomarkers for the metabolic syndrome, but also produce high levels of cytokines (promote vascular permeability) and cause desmoplasia, which together support tumour progression. At the same time, dendritic cells become chronically activated through the suppression of tolerogenic signalling pathways such as the canonical WNT and peroxisome proliferator-activated receptor-γ (PPARγ) pathways, eventually leading to T cell exhaustion. T cell activation is further suppressed by myeloid-derived suppressor cells (MDSCs), which engage co-inhibitory checkpoint molecules on T cells (such as programmed cell death 1; PD-1). Together, each of these cell types facilitates evolution of the tumour microenvironment and disease progression. MAPK, mitogen-activated protein kinase; TGFβ, transforming growth factor-β.