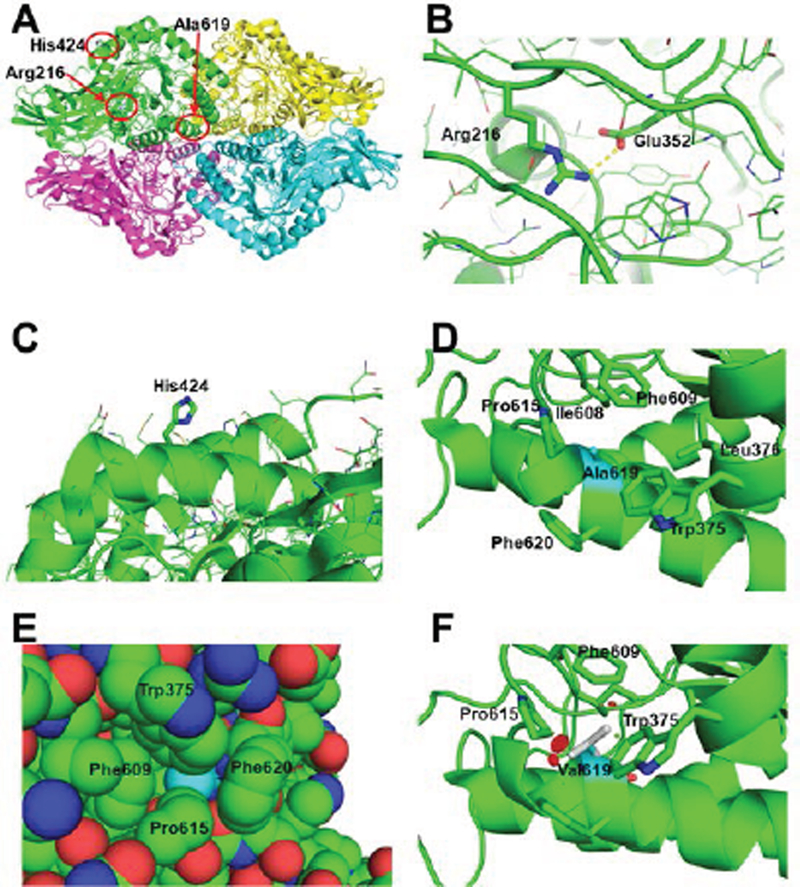
Figure 5. Structural basis for the GUSB defects.
A) The quaternary structure of human GUSB (PDB: 3HN3, Hassan et al., 2013), showing the regions of the Arg216Gln, His424Tyr and Ala619Val mutations in subunit A (green) highlighted by the red circles. B) Close up of the interaction of the Arg216 residue with Glu352 (illustrated as thicker sticks). The yellow dotted line represents a hydrogen bond (2.8 Å). The sidechains of other amino acids in the region are shown as line drawings. C) Zoom of the surface area of His424 (thick sticks) with the side chains of surrounding residues shown in line drawings. D) Close-up of the area of Ala619 (in cyan carbons, while other residues are with green carbons) in human GUSB. The neighboring residues producing the hydrophobic pocket containing Ala619 are shown with stick sidechains, including Trp375, Leu376, Ile608, Phe609, Pro615, and Phe620. E) van der Waals space filling representation of Ala619 (cyan) and surrounding residues, showing it is tightly packed inside the structure. F) One of three possible rotomers of the mutated Val619, showing clashes with surrounding residues (red disks), in this case Pro615, the main chain of the α-helix including Val619 and Phe609. The other two rotomers also clashed with other surrounding residues (not shown).