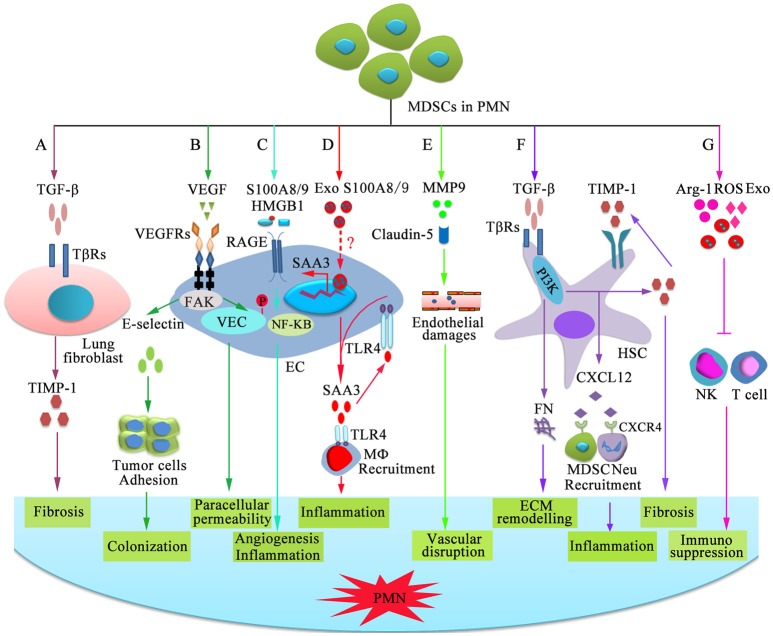
Figure 2.
Mechanisms of MDSC-dependent promotion of PMN formation and evolution. MDSC-derived factors participate in the stepwise evolution of the PMN through regulating local resident cells, resulting in a microenvironment that encourages the settlement and outgrowth of incoming cancer cells. (A) MDSCs stimulate lung fibroblasts to release tissue inhibitor of metalloproteinase 1 (TIMP1) by producing TGF-β, which promotes lung fibrosis. (B) VEGF-dependent induction of endothelial focal adhesion kinase (FAK) promotes E-selectin upregulation, which facilitates the adhesion of circulating tumor cells. VEGF triggers FAK-dependent vascular endothelial cadherin (VEC) phosphorylation in ECs and initiates paracellular permeability. (C) S100A8/9 and HMGB1 bind to RAGE on ECs and promote capillary-like tube formation and production of pro-inflammatory factor through the NF-κB signaling pathway, which is beneficial for angiogenesis and inflammation. (D) Exosomal S100A8/9 regulates SAA3 expression by ECs. SAA3 attracts macrophages to the pre-metastatic lungs through Toll-like receptor 4 (TLR4), which is beneficial for the formation of inflammatory microenvironment. (E) MMP9 damages the endothelial barrier of blood vessel through damaging tight junction protein claudin-5. (F) TGF-β induces fibronectin (FN) production and endogenous TIMP1 expression in hepatic stellate cells (HSCs) through phosphatidylinositol 3-kinase (PI3K). FN is conducive to tissue remodeling in the liver and initiate PMN formation. Moreover, circulating TIMP1-activated HSCs express C-X-C motif chemokine 12 (CXCL12), which induces MDSC and neutrophil migration through CXCR4 and creates a microenvironment in the liver that increases its susceptibility to tumor cells. (G) MDSCs suppress NK-and T-cell function by secreting immunosuppressive molecules and exosomes.