Abstract
Background and aim
More than 50% of the liver should be drained in case of unresectable hilar liver stenosis; however, it remains unclear if the use of several types of drainage (endoscopic retrograde cholangiography and pancreatography, percutaneous-biliary drainage, endoscopic ultrasound biliary drainage (EUS-BD)), allowing better drainage, has an impact on survival. The aim of our study was to evaluate the percentage of liver drained and its correlation on survival whatever the drainage technique used.
Patients and methods
This study was a retrospective analysis of a prospective registry of patients with malignant drainage stenosis of the hilum. The quality of drainage was evaluated based on the percentage of liver segments drained, which was calculated by dividing the number of liver segments drained by the total number of liver segments. Drainage could be achieved via an endoscopic, EUS-guided or percutaneous route not associated with the procedure.
Results
Sixty patients (38 men) were included from January 2015 to July 2016. The mean patient age was 69.84 years. Stenosis was classified as type II for 17 (29%) patients, type III for 20 (34%) patients, and type IV for 22 (37%) patients. Histology revealed cholangiocarcinoma for 26 (43%) patients, metastatic disease from colorectal cancer for 15 (25%) patients and another cancer for 19 (32%) patients. The median survival time was five (2.3–12.3) months.
The percentage of liver segments drained had a significant prognostic impact on overall survival regardless of the technique used to drain the liver. The percentage of liver segments drained was dichotomized based on a threshold value of 80%, resulting in two groups (<80% and ≥80%). Univariate analysis of overall survival revealed that the patients with <80% of liver segments drained had significantly worse prognoses (hazard ratio (HR) = 3.25 (1.66–6.36), p < 0.001) than the patients with ≥80% of liver segments drained. This effect was confirmed in multivariate analysis (HR = 2.46 (1.16–5.23), p = 0.02).
The other factor that affected survival was invasion of <50% of the liver by the tumor.
A receiver operating characteristic curve was used to establish a correlation between patients receiving chemotherapy and the percentage of liver drained (area under the curve = 0.77 (0.65–0.88)).
Conclusion
The survival of patients with malignant stenosis of the biliary confluence is highly correlated with the percentage of liver segments drained, regardless of the technique used.
Keywords: ERCP, hilar stenosis, palliative biliary drainage, biliary hilum stenting, EUS-guided biliary drainage of the liver hilum
Key summary
Current knowledge
Drainage of more than 50% of the liver improves survival.
Percutaneous drainage is recommended for type IV stenosis.
What is new
Survival continues to improve as liver drainage percentage increases beyond 50%.
Chemotherapy can be performed if the liver is well drained, and a good drainage percentage for chemotherapy is 71%.
Survival does not depend on the technique used to drain the liver, except in cases involving placement of an external drain.
Introduction
Biliary drainage has been a major component of the best supportive care for malignant hilar stenosis since the 2000s.1,2 The European Society of Gastrointestinal Endoscopy (ESGE) guidelines3 and an Asia-Pacific group4 recommend draining more than 50% of the liver based on a French study that showed longer overall survival for biliary drainage greater than 50% of the liver volume.5 The choice of the drainage technique is not standardized, and the ESGE did not comment on the choice of technique. Percutaneous drainage has fewer infectious complications but more noninfective complications (e.g. bleeding, pancreatitis). Recently, Wiggers et al.6 demonstrated that in complex hilar stenosis (type III/IV), percutaneous drainage is recommended.
Few data are available on the oncological follow-up of patients with respect to survival and potential chemotherapy. The aim of our study was to demonstrate that regardless of the drainage technique used, the goal of drainage is to drain the maximal amount of liver, even if multiple techniques must be used to realize benefits for overall survival and increase the possibility of performing palliative chemotherapy.
Patients and methods
Our study is a retrospective work based on prospective data and was performed according to an institutional review board agreement under the authority of the CNIL, the French regulatory body responsible for enforcing data privacy. Written, informed consent was obtained from each patient included in the study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee.
The study was conducted from January 2015 to July 2016 at a single center.
The inclusion criteria were unresectable, proven hilar cholangiocarcinoma or unresectable, highly suspicious hilar malignant stenosis without confirmative histological findings.
Exclusion criteria were benign stenosis, presurgical endoscopic drainage, blood coagulation disorder, or refusal of the patient to be included in a prospective registry. The retrospective analysis was performed in September 2016 using the hospital’s prospective, collected computerized patient file and, in the absence of recent data, by contacting patients’ physicians or referring gastroenterologists or the patients themselves.
None of the data reported here have been published previously.
Drainage was performed by five operators performing endoscopic retrograde cholangiography and pancreatography (ERCP), endoscopic ultrasound (EUS)-guided drainage, and percutaneous drainage. The choice of technique was at the discretion of the operators. Any technique could be chosen. In the case of hilar stenosis, the policy of the unit is to try to drain the majority of the liver segments. All drainage techniques were performed under general anesthesia on an intubated patient.
Definitions
A diagnosis of malignancy was based on histology, computed tomography (CT) scan or magnetic resonance imaging (MRI) and confirmed by the decision of a multidisciplinary team. Follow-up with disease progression confirmed the diagnosis of malignancy or benign stenosis in the case of no disease evolution after six months of follow-up.
Unresectability was determined with a CT scan or MRI performed before drainage. Therapeutic management was approved by a multidisciplinary team.
The quality of the drainage was defined by the percentage of liver segments drained.
This percentage was calculated by dividing the number of liver segments drained by the total number of liver segments. The number of liver segments was obtained by removing, from the eight classical liver segments, the segments resected in cases of surgery and/or the segments with 50% or more invasion by the tumor and/or any complete atrophic segments.
Endoscopic drainage could be performed in two or more sessions when planned because of complex stenosis. The intersession time period was required to be fewer than seven days (including nonworking days). Endoscopic reintervention was defined as the performance of a new endoscopic procedure seven or more days after the end of drainage.
A postoperative complication was defined as a complication that occurred during the first month after drainage. The definition of a complication was based on the consensus regarding surgical complications.7 Chemotherapy could be performed if the patient’s bilirubin level was <1.5 N.
Follow-up started on the date of endoscopic drainage and ended in September 2016 or at patient death, whichever occurred first.
The aim of the study was to evaluate the effect of drainage quality on the patients’ overall survival.
Drainage techniques
All procedures were performed on an intubated patient in the supine position.
ERCP
ERCP was performed as described in our previous paper and according to the ESGE guidelines.8,9
Stents were positioned transpapillary and using the side-by-side technique. A Pentax scope (ED-3490TK) was used with a CannulaTome from Cook Medical®; Jagwire™ 0.35-inch from Boston Scientific was used as the guidewire. A G-flex wire (0.35-inch, stiff, G-FLEX, Europe) was used. The dilation balloon was a Hurricane 4 mm (Hurricane Balloon Dilation Catheter 4 mm*4 cm, Boston Scientific). The stents used were Cook 8 and 10 cm, uncovered (evolution biliary stent system, uncovered, Cook Medical) and Taewoong 12 cm (Niti-S biliary uncovered stent-S-Type- 120*10 mm, Taewoong Medical).
EUS-guided hepaticogastrostomy
The EUS procedure was performed with a therapeutic echo-endoscope (EG38UTK (Pentax, Tokyo, Japan)) with large working channels of 3.8 mm, under triple guidance with ultrasound, endoscopic and fluoroscopic control. The echo-endoscope was positioned in the stomach. Liver segment II, or sometimes segment III, was punctured with an access needle 19G (EchoTip® Ultra 19-A, Cook Medical) or with a standard 19G needle (EchoTip® Ultra 19, Cook Medical). After opacification, a guidewire (Jagwire 0.35-inch from Boston Scientific) was introduced in the left bile duct. A fistula was created with a 6F cystotomy. A stent was then placed. A 6F nasobiliary drain was placed at the operator’s discretion.10,11
The stents inserted included a Giobor™ stent (10*80 mm and 10*100 mm Niti-S biliary covered stents, Giobor, Taewoong Medical) and a Poincloux stent (Hanarostent® partially covered biliary stent, 10*100 mm, MI Tech).
Percutaneous transhepatic drainage (PCTD)
After the insertion of a chiba needle (Neff percutaneous 21 G with access set with a hydrophilic coating and nitinol wire guide) and catheter (Cook Medical) included in the set, under US guidance, a hydrophilic wire was placed in the intrahepatic ducts (Jagwire 0.035-inch, Boston Scientific). Other guidewires, catheters and dilation balloons (Amplatz extra-stiff wire guide, 0.035-inch, Cook Medical; balloon dilation, percutaneous Zilver® Biliary Stent; external drain, Ultrathane; multipurpose drainage catheter, 8.5 Fr, Cook Medical) were used to cross the stenosis, to place the wire correctly in the bile ducts, and to insert the stents in the bile ducts. In case of the rendezvous technique, no external drain was placed. Stents were planned to be placed side by side.
After stent insertion, the flow of a contrast product and the presence of aerobilia were checked. All opacified liver segments were supposed to be drained.
Statistical analysis
All statistical analyses were performed at the significance level of α = 0.05 and with SAS® 9.3 software. Qualitative data were described by using counts and frequencies, and quantitative data were described by using means and standard deviations. Overall survival was defined as the duration from the date of the endoscopic drainage to the date of death or the last follow-up. Living patients were evaluated at the date of their last follow-up. Pointwise estimations were performed by the Kaplan-Meier method. Univariate Cox models were estimated to assess the influence of the following factors on overall survival: the presence of early complications, pathology (cholecystokinin, colon, other), hepatic invasion rate (<50% vs ≥50%), dilation and invasion of segment I, Klatskin classification (I–II, III, IV), bilirubin rate before drainage (<300 vs ≥300 ml), quantitative percentage of the drained liver segment, percentage of the drained liver segment (≥80% vs <80%), type of drainage (retrograde only, EUS+Trans Hepatic Biliary Drainage (THBD), retrograde + EUS +THBD), external drainage, number of endoscopic sessions (one or two to three), type of stent (metallic, plastic) and number of stents (one or several). A multivariate Cox model was then estimated including the factors identified as significant in the univariate analyses. For all Cox models, hazard ratios (HRs) were estimated with their bilateral confidence intervals (CIs).
The diagnostic performance of the percentage of liver segments drained on the possibility of administering chemotherapy and/or radiotherapy was assessed by a univariate logistic model. The area under the associated receiver operating characteristic (ROC) curve was estimated along with its bilateral Wald CI. The point corresponding to the minimal distance to the point of maximal sensitivity and specificity was estimated, and the percentage of liver segments was dichotomized by using this threshold. The impact of this new qualitative factor on the administration of chemotherapy and/or radiotherapy was then assessed by a univariate logistic model. The diagnostic performance of the percentage of liver segments drained on the necessity for a repeat endoscopy was assessed by a univariate logistic model. For all logistic regressions, odds ratios (ORs) were estimated with their bilateral Wald CIs.
Outcomes
From January 2015 to July 2016, a total of 65 patients were included. Five patients were excluded: One patient was excluded because the stenosis was diagnosed as benign at the follow-up, two patients were excluded because they were lost to follow-up, and two patients were excluded because endobiliary radiofrequency ablation was performed. As a result, 60 patients (38 men, mean age = 69.8 years) were analyzed.
Histology corresponded to cholangiocarcinoma for 26 patients (43%), metastatic disease from colorectal cancer for 15 patients (25%) and other metastatic etiologies for 19 patients (32%). The Bismuth classification of the stenosis was type II for 17 patients (29%), type III for 20 patients (34%), and type IV for 22 patients (37%).
The mean invasion of the liver was 21%; five patients presented with duodenal stenosis, and seven patients presented with ascites.
Forty patients were drained only by ERCP and the others by ERCP assisted with EUS drainage or PCTD.
The mean hospitalization length was 8.54 (±6.52) days. Postoperative morbidity was 52% (31 patients) and postoperative mortality was 23% (14 patients). Among the 14 patients who died during the postoperative month, 10 (71%) had duodenal stenosis, ascites, or more than 50% of the liver invaded by the tumor.
The median follow-up was 8.5 months (95% CI (5.5–16.2)). The median survival was 5.0 months (95% CI (2.3–12.3)). The percentage of liver segments drained had a significant prognostic impact on overall survival, with a higher percentage associated with a significantly better prognosis. For example, a 10-point difference in the percentage of liver segments drained (for values between 0% and 100%) was associated with a significant 24% survival benefit for patients with greater liver drainage (HR = 0.76 (0.67–0.87), p < 0.0001). As shown in Table 1, in univariate analyses, an early complication (HR = 2.42 (1.23–4.78), p = 0.01), liver invasion greater than 50% (HR = 4.68 (2.01–10.91), p < 0.001) and percentage of the drained liver less than 80% (HR = 3.25 (1.66–6.36), p < 0.001) were significantly worse prognostic factors for overall survival. In multivariate analysis, percentage of liver drainage <80% (HR = 2.46 (1.16–5.23), p = 0.02), liver invasion rate (HR = 2.90 (1.15–7.25), p = 0.02) and early complications (HR = 2.68 (1.32–5.44), p = 0.007) remained significantly associated with poor prognosis.
Table 1.
Univariate Cox regression analysis of overall survival.
| Effect | Contrast | Hazard ratio (95% CI) | Wald p value |
|---|---|---|---|
| Early complications | Presence vs absence | 2.42 (1.23, 4.78) | 0.011 |
| Diagnosis | Colon vs CCK | 1.75 (0.77, 3.94) | 0.180 |
| Other vs CCK | 1.44 (0.64, 3.25) | 0.374 | |
| Hepatic invasion rate | ≥50% vs <50% | 4.68 (2.01, 10.91) | <0.001 |
| Segment I | Dilated/invaded vs not invaded and not dilated | 1.37 (0.41, 4.58) | 0.606 |
| Klatskin classification | III vs I–II | 1.35 (0.54, 3.38) | 0.515 |
| IV vs I–II | 1.96 (0.83, 4.65) | 0.124 | |
| Bilirubin rate before drainage | ≥300 vs <300 | 0.89 (0.41, 1.90) | 0.760 |
| Percentage of drainage | Augmentation of 10% | 0.76 (0.67, 0.87) | <0.0001 |
| Percentage of drainage | <80% vs ≥80% | 3.25 (1.66, 6.36) | <0.001 |
| Type of drainage | EUS + PCTD vs retrograde only | 0.68 (0.32, 1.48) | 0.334 |
| Retrograde + EUS + PCTD vs retrograde only | 0.29 (0.04, 2.19) | 0.233 | |
| External drainage | Yes vs no | 0.73 (0.28, 1.93) | 0.527 |
| No. of endoscopic sessions | 1 vs 2–3 | 2.98 (1.04, 8.58) | 0.042 |
| Type of stent | Plastic vs metallic | 0.48 (0.17, 1.38) | 0.174 |
| Number of stents | Several vs one | 0.60 (0.33, 1.31) | 0.232 |
CCK: Cholangiocarcinoma; CI: confidence interval; EUS: endoscopic ultrasound; PCTD: percutaneous transhepatic drainage.
The ROC curve associated with the diagnostic performance of the percentage of liver segments drained on the administration of chemotherapy and/or radiotherapy is displayed in Figure 1. The area under the curve favored high-quality drainage (0.77 (0.65, 0.88)), which was confirmed by the associated logistic model (OR = 1.06 (1.02, 1.09), p = 0.0007). According to the ROC curve, a percentage of liver segments drained greater than or equal to 71% is a significant predictor of the administration of chemotherapy and/or radiotherapy (92% vs 44%, OR = 0.06 (0.01, 0.32), p = 0.0008). A postoperative complication negatively predicted the performance of chemotherapy: Nineteen (73%) of the 26 patients who received chemotherapy and/or radiotherapy did not have a postoperative complication (p = 0.004, chi-squared test).
Figure 1.
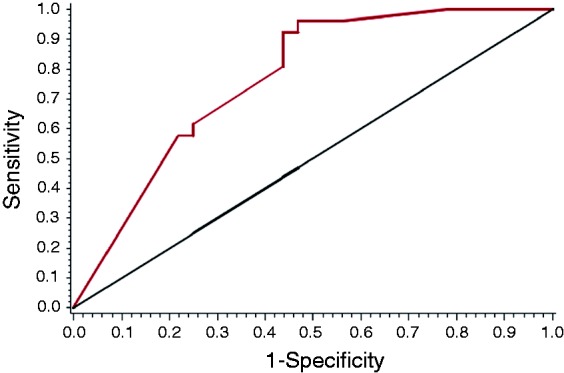
Chemotherapy (CT) or radiotherapy (RT) received by the patients and correlation with the percentage of liver segment drained. ROC: receiver operating characteristic.
Cholangitis, which requires repeat endoscopy during follow-up, affected 28 (45.9%) of the patients. There was a tendency toward a correlation between a high percentage of drained liver and a low rate of endoscopic reintervention (OR = 1.02 (0.99, 1.05), p = 0.06). Endoscopic reintervention was significantly higher in cases of external drains placed initially (p = 0.02, Fisher exact test); however, only eight patients had external drains, of whom seven needed a repeat endoscopy.
Other data are available in Tables 2–6.
Table 2.
Characteristics of endoscopic procedures.
| Characteristic | n (%) |
|---|---|
| Type of drainage | |
| Retrograde drainage only | 40 (67%) |
| EUS drainage only | 2 (3%) |
| ERCP + EUS | 4 (7%) |
| ERCP + PCTD | 10 (17%) |
| PCTD only | 1 (2%) |
| ERCP + PCTD + EUS | 3 (5%) |
| Type of stent | |
| Plastic stents | 12 (21%) |
| Metallic stents | 46 (79%) |
| External drain | 8 (13%) |
| Number of stents | |
| 1 | 20 (33%) |
| 2 | 32 (53%) |
| 3 | 7 (12%) |
| 4 | 1 (2%) |
| Endoscopic sessions required | |
| 1 | 49 (82%) |
| 2 | 9 (15%) |
| 3 | 2 (3%) |
ERCP: endoscopic retrograde cholangiography and pancreatography; EUS: endoscopic ultrasound; PCTD: percutaneous transhepatic drainage.
Table 3.
Early complications (31 patients).
| Characteristic | Category | n (%) |
|---|---|---|
| Etiologies | Sepsis | 10 (32%) |
| Progression of the disease with rapid deterioration of the patient without another obvious cause other than disease progression | 8 (26%) | |
| Under capsular hematoma | 3 (10%) | |
| Pulmonary embolism | 2 (6%) | |
| Duodenal perforation due to plastic stent migration | 1 (3%) | |
| Pancreatitis | 4 (13%) | |
| Hemobilia | 2 (6%) | |
| Fecaloma with intestinal occlusion | 1 (3%) | |
| Duodenal involvement or ascites | Presence | 7 (23%) |
| Hepatic invasion ≥50% | 8 (26%) | |
| Pathology | Cholecystokinin | 13 (42%) |
| Gallbladder adenocarcinoma | 2 (6%) | |
| Colon cancer | 7 (23%) | |
| Other metastasis | 9 (29%) |
Table 4.
Etiologies of postoperative mortality (14 patients).
| n (%) | |
|---|---|
| Pathology | |
| Cholecystokinin | 6 (43%) |
| Gallbladder adenocarcinoma | 1 (7%) |
| Colon cancer | 1 (7%) |
| Other metastasis | 6 (43%) |
| Duodenal involvement or ascites | |
| Presence | 5 (36%) |
| Absence | 9 (64%) |
| Hepatic invasion >50% | 5 (35%) |
| Etiologies | |
| Sepsis | 4 (29%) |
| Progression of the disease with rapid deterioration of the patient without another obvious cause other than disease progression | 7 (50%) |
| Under capsular hematoma | 2 (14%) |
| Pulmonary embolism | 1 (7%) |
Table 5.
Pathologies of the stenosis.
| Pathology | n (%) | Histology obtained |
|---|---|---|
| Cholangiocarcinoma | 26 (43%) | 13/26 (50%) |
| Colorectal adenocarcinoma | 15 (25%) | 100% |
| Breast cancer | 5 (8%) | 100% |
| Pancreatic adenocarcinoma | 2 (3%) | 100% |
| Endocrine pancreatic tumors | 2 (3%) | 100% |
| Gallbladder carcinoma | 2 (3%) | 100% |
| Lung cancer | 2 (3%) | 100% |
| Ovarian cancer | 1 (2%) | 100% |
| Lymphoma | 1 (2%) | 100% |
| Hepatocarcinoma | 1 (2%) | 100% |
| Gastric adenocarcinoma | 1 (2%) | 100% |
| Renal cancer | 1 (2%) | 100% |
| Cardiac | 1 (2%) | 100% |
Table 6.
Multivariate Cox regression analysis of overall survival.
| Effect | Contrast | Hazard ratio (95% CI) | Wald p value |
|---|---|---|---|
| Early complications | Presence vs absence | 2.68 (1.32, 5.44) | 0.007 |
| Hepatic invasion rate | ≥50% vs <50% | 2.90 (1.15, 7.28) | 0.02 |
| Percentage of drainage | <80% vs ≥80% | 2.46 (1.16, 5.23) | 0.02 |
| No. of endoscopic sessions | 1 vs 2–3 | 2.28 (0.79, 6.55) | 0.13 NS |
CI: confidence interval.
Discussion
To our knowledge, our study is the first to evaluate the percentage of the drained liver and its correlation with the possibility of administering chemotherapy and patient survival.
Hilar drainage is challenging and demanding. When attempting to place multiple stents, the risk of morbidity increases, mainly from the risk of leaving an opacified liver segment.12,13 A strength of our study is that all operators performed all drainage techniques. Few studies have involved combining several drainage techniques, and in certain investigations, a combination of drainage techniques was used only as salvage therapy to drain opacified segments.14,15
Although the rate of technical failure of endoscopic drainage remains as high as 20%,16,17 the use of a combination of several drainage techniques allowed an average of 75% of the liver to be drained. Surprisingly, we did not find any difference in overall survival regardless of the technique used, although more receptive endoscopy for cholangitis was performed when an external drain was temporarily left in place. This absence of a difference was seen in spite of the higher morbidity described in cases of PCTD or EUS drainage. This finding underlines the importance of obtaining high-quality drainage; however, rendezvous techniques are preferred in cases of PCTD to avoid external drainage.
Hilar biliary drainage for hilar tumors is a palliative treatment. However, palliative treatments, including chemotherapy and radiotherapy, have not previously been studied and correlated with drainage quality. Another important finding of our study is the evidence that correctly drained livers allow the patients to receive chemotherapy, which correlates with survival.
We observed a high mortality rate (14%). Complications have not been well described in the literature. Our rates of endoscopic reintervention for cholangitis, postoperative morbidity and mortality may be high but are comparable to those reported in the literature. Global complications (postoperative complications and repeat endoscopy) of up to 81% were described by Liberato and Canena,18 and mortality rates of 16% to 18% were described by Deviere and colleagues19 and Iwano et al.20
Mortality is also associated with advanced disease before hilar drainage (10 patients had duodenal stenosis, ascites, or more than 50% of the liver invaded by the tumor). As a result, biliary drainage should be maximal except in patients with advanced disease, as described previously. As previously reported, in this case, drainage of only 25% of the liver could be discussed.21 We can explain our high rate of mortality based on our definitions of morbidity and mortality.7 Complications until one month after the procedure were considered in this investigation, whereas certain studies described complications only up to 15 days after surgery. Moreover, our mortality rate may be attributable to the choice to drain patients with advanced disease. It is likely that a minimalist approach to drainage could be employed in cases of advanced disease (liver invasion >50%, ascites, and duodenal stenosis).
One limitation of this study is its retrospective design. However, it was an observational study. The main limitation in the data due to the retrospective design is the regression of the patients with postoperative complications. It is likely that the evolution of complications was made worse because of the poor condition of the patients due to their disease.
Another limitation could be the evaluation of the drained liver. For example, segment I was discussed because dilation of segment I exists, and we would like to know whether it was a negative predictive factor. Volumetric analysis was not performed because such analysis is difficult to conduct in current practice. Score calculation was performed by only one operator to limit bias and should therefore be checked by other operators and by volumetric calculation.
Conclusion
The percentage of the liver drained in the endoscopic management of palliative malignant hilar stenosis is essential. The more segments of the liver that are drained, the more likely it is that the patient will be able to undergo chemotherapy. The percentage of the liver drained also correlates with survival.
Declaration of Conflicting Interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Informed Consent
Written, informed consent was obtained from each patient included in this study.
Ethics Approval
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee.
References
- 1.Smith AC, Dowsett JF, Russel RC, et al. Randomized trial of endoscopic stenting versus surgical bypass in malignant low bile duct obstruction. Lancet 1994; 344: 1665–1660. [DOI] [PubMed] [Google Scholar]
- 2.Deviere J, Baize M, de Toeuf J, et al. Long-term follow-up of patients with hilar malignant stricture treated by endoscopic internal biliary drainage. Gastrointest Endosc 1998; 34: 95–101. [DOI] [PubMed] [Google Scholar]
- 3.Dumonceau JM, Tringali A, Blero D, et al. Biliary stenting: Indications, choice of stents and results: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy 2012; 44: 277–292. [DOI] [PubMed] [Google Scholar]
- 4.Rerknimitr R, Angsuwatcharakon P, Ratanachu-ek T, et al. Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J Gastroenterol Hepatol 2013; 28: 593–607. [DOI] [PubMed] [Google Scholar]
- 5.Vienne A, Hobeika E, Gouya H, et al. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: The role of liver volume assessment. Gastrointest Endosc 2010; 72: 728–735. [DOI] [PubMed] [Google Scholar]
- 6.Wiggers JK, Groot Koerkamp B, Coelen RJ, et al. Preoperative biliary drainage in perihilar cholangiocarcinoma: Identifying patients who require percutaneous after failed endoscopic drainage. Endoscopy 2015; 47: 1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complication. Five-year experience. Ann Surg 2009; 250: 187–196. [DOI] [PubMed] [Google Scholar]
- 8.Caillol F, Bories E, Auttret A, et al. Evaluation of PCLE in the bile duct: Final results of EMID study: pCLE: Impact in the management of bile duct strictures. Surg Endosc 2015; 29: 266–268. [DOI] [PubMed] [Google Scholar]
- 9.Testoni PA, Mariani A, Aabakken L, et al. Papillary cannulation and sphincterotomy techniques at ERCP: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy 2016; 48: 657–683. [DOI] [PubMed] [Google Scholar]
- 10.Bories E, Pesenti C, Caillol F, et al. Transgastric endoscopic ultrasonography-guided biliary drainage: Results of a pilot study. Endoscopy 2007; 39: 287–291. [DOI] [PubMed] [Google Scholar]
- 11.Poincloux L, Rouquette O, Buc E, et al. Endoscopic ultrasound-guided biliary drainage after failed ERCP: Cumulative experience of 101 procedures at a single center. Endoscopy 2015; 47: 794–801. [DOI] [PubMed] [Google Scholar]
- 12.Chang WH, Kortan P, Haber GB. Outcome in patients with bifurcation tumors who undergo unilateral versus bilateral hepatic duct drainage. Gastrointest Endosc 1998; 47: 354–362. [DOI] [PubMed] [Google Scholar]
- 13.Paik WH, Park YS, Hwang JH, et al. Palliative treatment with self-expandable metallic stents in patients with advanced type II or IV hilar cholangiocarcinoma: A percutaneous versus endoscopic approach. Gastrointest Endosc 2009; 69: 55–62. [DOI] [PubMed] [Google Scholar]
- 14.Ogura T, Onda S, Takagi W, et al. Clinical utility of endoscopic ultrasound-guided biliary drainage as a rescue of re-intervention procedure for high-grade hilar stricture. J Gastroenterol Hepatol 2017; 32: 163–168. [DOI] [PubMed] [Google Scholar]
- 15.Jang SI, Hwang JH, Lee KH, et al. Percutaneous biliary approach as a successful rescue procedure after failed endoscopic therapy for drainage in advanced hilar tumors. J Gastroenterol Hepatol 2017; 32: 932–938. [DOI] [PubMed] [Google Scholar]
- 16.Lee SH, Park JK, Yoon WJ, et al. Optimal biliary drainage for inoperable Klatskin’s tumor based on Bismuth type. World J Gastroenterol 2007; 13: 3948–3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saluja SS, Gulati M, Garg PK, et al. Endoscopic or percutaneous biliary drainage for gallbladder cancer: A randomized trial and quality of life assessment. Clin Gastroenterol Hepatol 2008; 6: 944–950.e943. [DOI] [PubMed] [Google Scholar]
- 18.Liberato MJ, Canena JM. Endoscopic stenting for hilar cholangiocarcinoma: Efficacy of unilateral and bilateral placement of plastic and metal stents in a retrospective review of 480 patients. BMC Gastroenterol 2012; 12: 103–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deviere J, Baize M, de Toeuf J, et al. Long-term follow-up of patients with hilar malignant stricture treated by endoscopic internal biliary drainage. Gastrointest Endosc 1988; 34: 95–101. [DOI] [PubMed] [Google Scholar]
- 20.Iwano H, Ryozawa S, Ishigaki N, et al. Unilateral versus bilateral drainage using self-expanding metallic stent for unresectable hilar biliary obstruction. Dig Endosc 2011; 23: 43–48. [DOI] [PubMed] [Google Scholar]
- 21.Dowsett JF, Vaira D, Hatfield AR, et al. Endoscopic biliary therapy using the combined percutaneous and endoscopic technique. Gastroenterology 1989; 96: 1180–1186. [DOI] [PubMed] [Google Scholar]


