Figure 3.
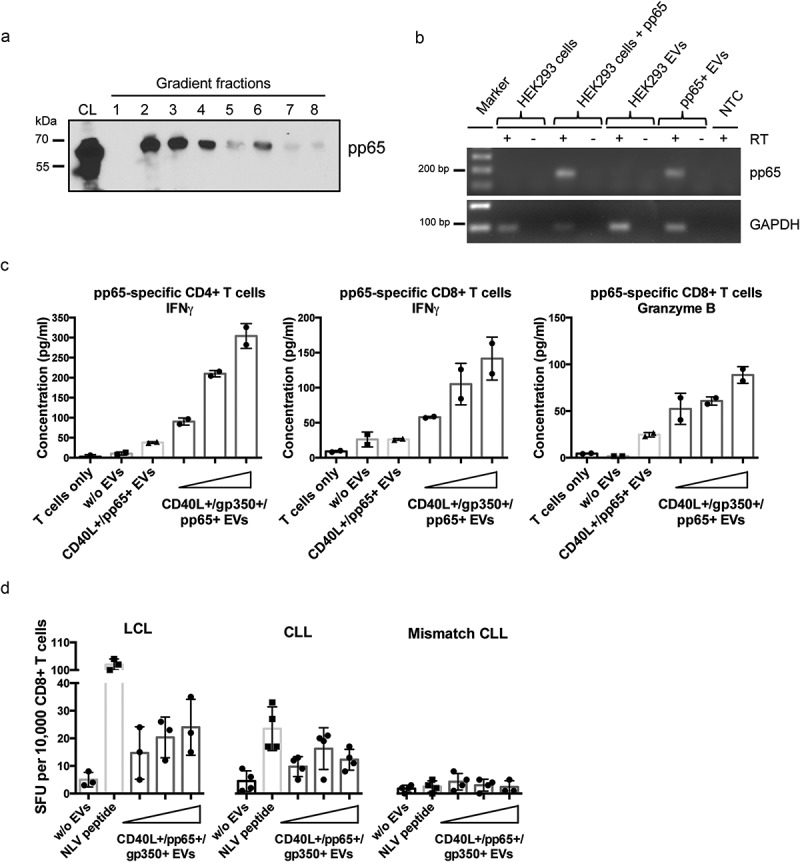
CMV pp65 is incorporated into HEK293-derived EVs and induces specific CD4+ and CD8+ T-cell responses.
(a) HEK293 cells were transiently transfected with a pp65 expression plasmid and EVs were isolated 3 days later by serial centrifugation and density gradient fractionation. Eight fractions were collected and analysed for pp65 by Western blotting. CL: cell lysate. (b) EVs derived from HEK293 cells expressing pp65 contain pp65 mRNA, as confirmed by reverse transcriptase (RT-) PCR using pp65-specific primers and cDNA isolated from HEK293 cells and the corresponding EVs. GAPDH mRNA was detected in both EVs from untransfected and transfected HEK293 cells. RT: reverse transcriptase; NTC: no template control. (c) 10,000 HLA-DQB1+ mini-LCLs were incubated overnight with 1000 ng of different types of EVs or left untreated, as indicated. CD40L+/gp350+/pp65+ EVs were applied in three different concentrations (250 ng, 500 ng, 1000 ng). EV-loaded mini-LCLs were co-cultured with an HLA-DQB1-restricted pp65-specific CD4+ T-cell clone at a 1:1 ratio. After 24 h, IFN-γ secretion was measured by ELISA. Middle and right diagram: HLA-A2+ LCLs were used to stimulate an HLA-A2-restricted pp65-specific CD8+ T-cell clone. The same settings as for CD4+ T cells were used. The concentrations of IFN-γ (middle) and granzyme B (right) in the supernatants were measured by ELISA. (d) LCLs or primary CLL cells isolated from an HLA-A2+ CMV-negative donors were loaded with increasing amounts of CD40L+/gp350+/pp65+ EVs and co-cultured on IFN-γ ELISpot filter plates with a HLA-A2-restricted pp65-specific CD8+ T-cell clone (10,000 cells/well) at a 1:1 ratio for 24 h. Wells containing T cells co-cultured with unloaded APCs or HLA-mismatched CLL cells were used as controls to confirm the specificity of the assay. Mean +SD of IFN-γ spot-forming units (SFU) per 10,000 T cells was enumerated.
