Keywords: nerve regeneration, secondary spinal cord injury, telemetry, pathological mechanism, rabbit, conscious, anesthetized, hemorrhage, edema, pressure measurement, blood-spinal barrier, neural regeneration
Abstract
Intramedullary pressure increases after spinal cord injury, and this can be an important factor for secondary spinal cord injury. Until now there have been no studies of the dynamic changes of intramedullary pressure after spinal cord injury. In this study, telemetry systems were used to observe changes in intramedullary pressure in the 72 hours following spinal cord injury to explore its pathological mechanisms. Spinal cord injury was induced using an aneurysm clip at T10 of the spinal cord of 30 Japanese white rabbits, while another 32 animals were only subjected to laminectomy. The feasibility of this measurement was assessed. Intramedullary pressure was monitored in anesthetized and conscious animals. The dynamic changes of intramedullary pressure after spinal cord injury were divided into three stages: stage I (steep rise) 1–7 hours, stage II (steady rise) 8–38 hours, and stage III (descending) 39–72 hours. Blood-spinal barrier permeability, edema, hemorrhage, and histological results in the 72 hours following spinal cord injury were evaluated according to intramedullary pressure changes. We found that spinal cord hemorrhage was most severe at 1 hour post-spinal cord injury and then gradually decreased; albumin and aquaporin 4 immunoreactivities first increased and then decreased, peaking at 38 hours. These results confirm that severe bleeding in spinal cord tissue is the main cause of the sharp increase in intramedullary pressure in early spinal cord injury. Spinal cord edema and blood-spinal barrier destruction are important factors influencing intramedullary pressure in stages II and III of spinal cord injury.
Chinese Library Classification No. R459.9; R364; R605
Introduction
Spinal cord injury (SCI) is a serious central nervous system disorder, and the global incidence of traumatic SCI is approximately 23 per million (Lee et al., 2014). The high disability rate of SCI seriously affects the quality of life of patients (Chen et al., 2016; Kreinest et al., 2016; Yang et al., 2017), and there is currently no effective cure. Spinal cord hemorrhage and edema occur rapidly after SCI, and intramedullary pressure (IMP) increases significantly when the dura mater is intact, then aggravating ischemia and hypoxia of the spinal cord (Saadoun et al., 2008; Werndle et al., 2014; Noussitou et al., 2015). These factors are strongly associated with the poor prognosis for neurological recovery after SCI (Miyanji et al., 2007; Batchelor et al., 2011). Spinal cord decompression after SCI can reduce IMP, thereby preventing secondary injury and improving prognosis (Yang et al., 2013; Hu et al., 2015). Thus, increased IMP may be an important pathophysiological mechanism of secondary injury after SCI, causing the primary lesion areas to expand (Oyinbo, 2011; Borgens and Liu-Snyder, 2012). In-depth study of the pathophysiology of SCI is an important pathway to finding new treatment strategies.
The timing of decompression is one of the key factors influencing the outcome of SCI (Liu et al., 2015; Furlan et al., 2016; Dakson et al., 2017; Piazza and Schuster, 2017). Understanding the physiological, biological, and functional changes that occur after SCI through IMP may help determine the appropriate time for decompression. However, the regulation of changes in IMP remains unclear, which is a major obstacle to applying decompression of the spinal cord in clinical settings.
Pressure measurement is the key to obtaining IMP regulation. However, there is no standard method for monitoring IMP after SCI. Most studies have measured IMP only in anesthetized animals (Jones et al., 2012; Soubeyrand et al., 2013; Leonard et al., 2015; Martirosyan et al., 2015; Dong et al., 2016; Khaing et al., 2017). Anesthesia may affect blood pressure in animals causing an indirect effect on IMP (Kwon et al., 2009). Additionally, previous studies have provided information at a single time point or during a specific period of time (only several hours). Therefore, the results from studies to date do not sufficiently describe the dynamic changes in IMP after SCI.
Wireless telemetry systems may enable studies that compensate for the shortcomings or previous investigations by allowing long-term real-time monitoring in conscious animals. Physiological signals over extended periods of time can be recorded in unstressed animals (i.e., unhindered by anesthesia or restraint). This approach has now been used to study cardiovascular function in rats (Hou et al., 2014), renal tissue oxygen tension (Koeners et al., 2016), and intracranial pressure (Guild et al., 2015).
In this study, IMP after SCI was monitored in both conscious and anesthetized rabbits using telemetry systems, focusing on the dynamic changes in IMP in conscious rabbits within the first 72 hours post-SCI. Moreover, permeability of the blood spinal cord barrier, edema, hemorrhage, and the tissue architecture of the spinal cord were investigated to identify the factors influencing IMP and to facilitate the development of new methods for the treatment of SCI.
Materials and Methods
Animals
Sixty-two healthy female Japanese white rabbits weighing 2.0 ± 2.5 kg and aged 2.5 ± 3.0 months were obtained from Beijing Jinmuyang Experimental Animal Breeding Co., Ltd. (Beijing, China; license No. SCXK [jing] 2015-0005). To fulfill the inclusion criteria, animals had to have no clinical signs of an underlying disorder of the spinal cord and all animals had normal spinal cords as assessed by standard magnetic resonance imaging protocols under anesthesia (Liu et al., 2018). The exclusion criteria were as follows: any suspicion of a neurological disorder affecting the spinal cord, any history of spinal cord disease, or any previous spinal cord surgery. All animals were kept in a 12-hour day/night environment and were allowed free access to food and water.
All the animal procedures were approved by the Experimental Animal Welfare Committee of Capital Medical University, China (approval number: AEEI-2018-008) on January 8, 2018.
Telemetry system
Briefly, the main system components included an implantable telemeter fitted with a Millar solid state pressure sensor (TRM5*, Millar Instruments, Houston, TX, USA) at the end of the catheter, allowing highly accurate measurement of pressure signals in the tip location. The telemeter weighed approximately 12.5 g and was encapsulated in biocompatible silicon and placed subcutaneously in each rabbit’s back. The telemeter sampled the current at 2000 Hz and transmitted data wirelessly on a dedicated frequency in the 2.4 GHz band to a remote receiving station. The system provided continuous operation enabled by battery recharging through inductive power transfer and the use of a pad placed under the floor of the rat cage (SmartPad TR181, Millar Instruments). The specific attachment procedures are described in detail elsewhere (Koeners et al., 2016). The telemetry system could provide two signals: pressure and temperature. Only the pressure (IMP) was of interest in this study.
SCI modeling
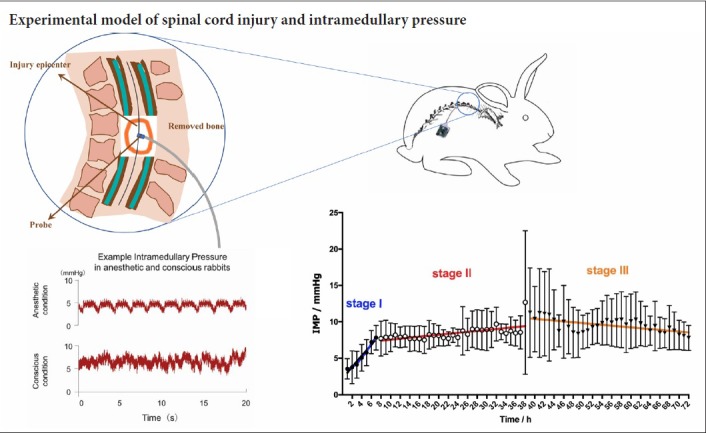
All 62 healthy Japanese white rabbits underwent laminectomy, and 30 underwent clip compression SCI at T10 (Figure 1). Before the experiment, the rabbits had to fast for 12 hours. Intravenous injection of pentobarbital (3%, 30 mg/kg, Sigma-Aldrich, Shanghai, China) was administered as an anesthetic. Taking T11 as the center, a longitudinal incision was made at approximately 3 cm to expose the T10–11 spinal cord (Liu et al., 2018). An aneurysm clip (REBSTOCK, Dürbheim, Tuttlingen, Germany) was used to laterally clamp T10 of the spinal cord, with an intensity of 90 g and a retention time of 1 minute (Fehlings and Tator, 1995). The spinal cord swelled and circular congestion occurred immediately after clamping (Figure 2).
Figure 1.
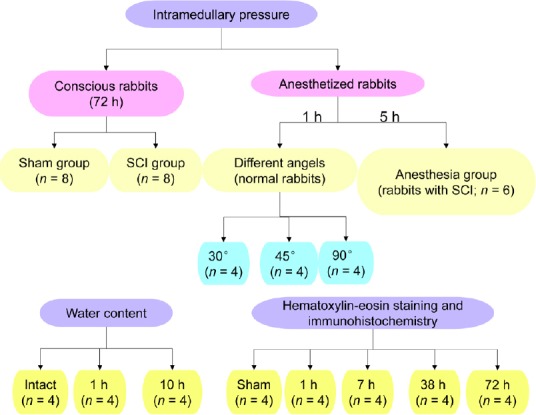
Experimental groups.
SCI: Spinal cord injury; h: hour(s).
Figure 2.
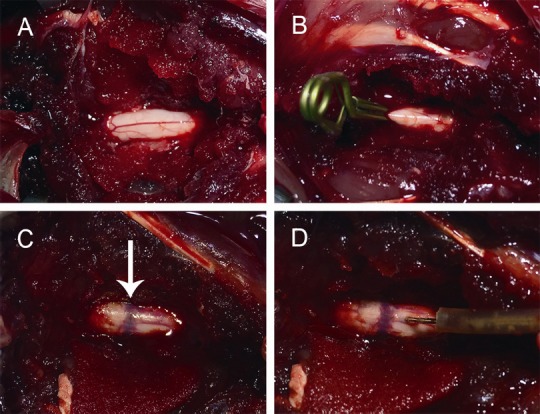
Illustration of surgical procedure.
(A) Rabbits undergo laminectomy. After removing the T10–11 spinous process and lamina, the spinal cord was exposed. (B) Using an aneurysm clip to laterally clamp the T10 segment of the spinal cord, which resulted in spinal cord injury at T10 segment. (C) Spinal cord swelling and circular congestion (arrow) occurred immediately after the clamping. (D) The pressure probe was inserted into the spinal cord.
The criteria for the development of a successful model were as follows: After the injury, bleeding and edema of the spinal cord occurred and the rabbits showed a tail-wagging reflex with retraction-like fluttering of the lower extremities and body; and upon the return of consciousness after anesthesia, the animal had flaccid paralysis in both hind legs (Renfu et al., 2014).
Measurement of IMP
Monitoring IMP in anesthesia group
Given that rabbits in the SCI group were fully awake 3–4 hours after surgery, rabbits in the anesthesia group (n = 6) were continuously anesthetized for 5 hours. To identify the effects caused by anesthesia, the IMPs at 5 hours were analyzed to compare the differences between conscious (SCI group) and anesthetized animals post-SCI.
Monitoring IMP in different probe angles
To assess any differences in IMP when the pressure probe was inserted into the spinal cord at different angles using a stereotaxic instrument (Huaibei Zhenghua Biologic Apparatus Facilities Co., Ltd., Anhui, China), rabbits were assessed for IMP stratified into 30° (n = 4), 45° (n = 4), and 90° groups (n = 4) as previously described (Dong et al., 2016), and the IMP recorded for 1 hour (Figure 3).
Figure 3.
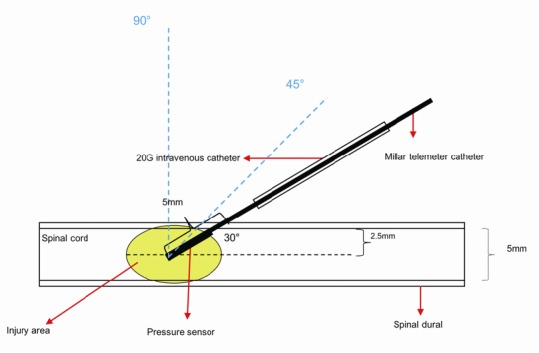
The pressure probe is inserted into the spinal cord at three different angles.
The spinal cord has a diameter of 5 mm. A 20 G intravenous catheter was used to penetrate the dura with a hole and avoid damaging the blood vessels of the spinal cord. The trocar was then withdrawn, and the pressure probe was inserted along the cannula to the center of the spinal cord. The length of the pressure catheter inside of the spinal dura was 5 mm. The angle of the probe shown to the spinal cord is 30°. Dotted lines indicate angles of 45° and 90°.
Monitoring in conscious rabbits
Ultra-early treatment of SCI has received much attention, especially within 72 hours after SCI (Phang et al., 2016; Matsushita et al., 2017; Chen et al., 2018). So to investigate the change in IMP after SCI, rabbits were assessed for IMP within 72 hours after SCI (SCI group, n = 8) or laminectomy (sham group, n = 8) using the Millar telemetry systems. After T10 SCI, the T11 dura was gently lifted with microscopic tweezers. A small hole was punctured with a needle. The probe was carefully inserted through the hole into spinal cord and moved to the epicenter of the injury in the SCI group, or moved to the T10 level in the sham group. Tissue glue (B. Braun Surgical, SA, Rubi, Spain) was used to fix it to avoid movement of the probe and leakage of the cerebrospinal fluid. The IMPs were monitored and recorded using Power Lab (AD Instruments, Sydney, Australia).
Neurologic assessment
Neurologic function was only assessed after IMP monitoring, and independently graded by two investigators without knowledge of the treatment. The motor function of the hind limbs was graded using the modified Tarlov scale (Baba et al., 2010) (5 = normal hop, 4 = weak hop, 3 = sits alone, 2 = sits with assistance, 1 = slight movement, 0 = no movement).
Water content of the spinal cord
To assess the safety of this method, rabbits without SCI were assessed for edema at 1 hour (n = 4) and 10 hours (n = 4) after probe insertion and compared with intact animals (without insertion, n = 4) using the wet weight/dry weight method as previously described (Vink et al., 2003). The rabbits were sacrificed by over-anesthesia with pentobarbital sodium (3%, 100 mg/kg). One-centimeter sections were dissected and weighed to obtain the wet weight. The spinal cord sections were then dried at 100°C for 48 hours and reweighed to obtain the dry weight. The percentage of the water content in the spinal cord was calculated using the following formula: % water =(wet weight − dry weight)/wet weight × 100.
Histological analysis and immunohistochemistry
To explore the factors affecting IMP, histological examination was carried out during IMP changes. Rabbits were deeply anesthetized (3%, 100 mg/kg) using pentobarbital sodium at 1 hour (n = 4), 7 hours (n = 4), 38 hours (n = 4), or 72 hours (n = 4) post-SCI. Spinal cord tissue was then processed, cut, and stained with hematoxylin and eosin for histopathological and morphological observation. Immunohistochemical analysis of spinal cord lesions was conducted by incubating sections overnight at 4°C with primary antibodies against anti-albumin (dilution 1:1000; rabbit albumin antibody, A120-104F; BETHLY, Montgomery, TX, USA) and anti-aquaporin 4 (AQP4) (1:100; Ab9512; Abcam, Cambridge, MA, USA). The sections were immerged in sodium citrate and heated to boiling in a microwave before incubation. After three washes with phosphate-buffered saline 0.2% (each for 5 minutes), the sections were incubated with conjugated goat anti-rabbit IgG (074-1506, KLP; Beijing XMJ Scientific Co., Ltd., Beijing, China) and goat anti-mouse IgG (074-1806; KLP, Beijing XMJ Scientific Co., Ltd.) for 1 hour at room temperature, immediately followed by administration of one to two drops of permanent mounting medium, and covering with a glass coverslip. All sections were digitally scanned at high resolution using HistoFAXS 3.0 (TissueGnostics, Vienna, Austria). The acquired images were viewed using the associated proprietary viewing software (FAXS viewer, TissueGnostics, Vienna, Austria). Photoshop CC (Adobe, San Jose, CA, USA) and ImageJ (National Institutes of Health, Bethesda, MD, USA) softwares were used to process and analyze images.
To determine blood volume, 5 μm serial sections were cut 500 μm apart and stained with hematoxylin and eosin. These were similarly digitally scanned and viewed using HistoFAXS, with the hemorrhagic regions calculated using the Cavalieri method defined as volume = ∑A·ISF ·t, where A is the area, ISF is the inverse of the sampling fraction, and t is the section thickness (Leonard et al., 2015). Densitometry was used to assess the intensity of AQP4 staining. Whole cross-sections were exported and albumin immunoreactivity was assessed using a color deconvolution method as previously described (Leonard et al., 2013; Varghese et al., 2014). DABwt% values obtained represent an estimate of the amount of 3,3′ diaminobenzidine (DAB and thus antigen) on the original tissue section. DABwt% was calculated with the following equation.
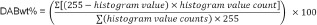
Statistical analysis
All data, expressed as the mean ± standard deviation, were analyzed using GraphPad Prism 7.0 (GraphPad Software Inc., San Diego, CA, USA). IMP in three probe angles, water content, blood volume, albumin immunoreactivity, and AQP4 were analyzed using a one-way analysis of variance followed by Tukey’s post hoc tests. Unpaired t-tests were used to compare IMP at 5 hours between conscious and anesthetized rabbits. The IMP between sham and SCI groups were analyzed using two-way repeated measures analysis of variance followed by Sidak’s multiple comparisons test (Bazett-Jones et al., 2017). The relationship between time and IMP in the SCI group was analyzed using linear regression analysis. A value of P < 0.05 was considered statistically significant.
Results
IMP in anesthesia group
IMP was recorded using real-time monitoring, and the average IMP per hour calculated, representing the IMP at each time point (hours). The IMP at 5 hours in anesthesia and SCI groups were 3.43 ± 1.33 mmHg and 5.76 ± 1.80 mmHg, respectively. There was a significant difference between these two groups (P < 0.05).
IMP in different probe insertion angles
The IMP at the three angles measured (30°, 45°, and 90°) was 3.79 ± 0.58 mmHg, 3.23 ± 0.95 mmHg, and 3.32 ± 0.32 mmHg, respectively. There was no significant difference between the three angles (P > 0.05). The average IMP was 3.45 ± 0.66 mmHg in normal anesthetized rabbits.
IMP in conscious rabbits
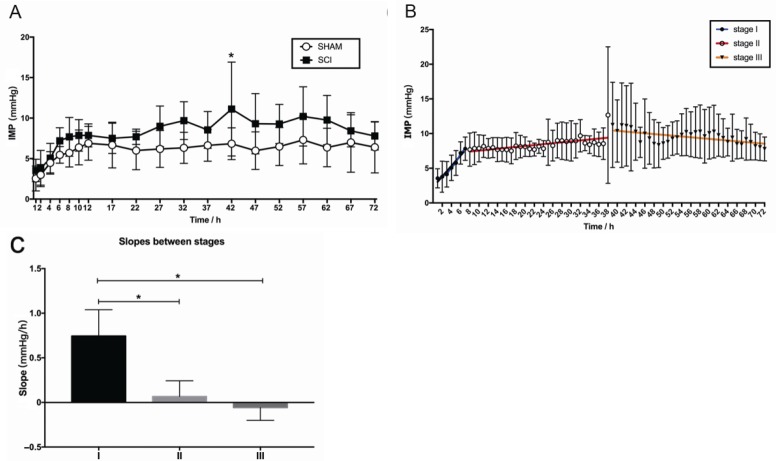
IMP in the sham group and SCI group were 5.93 ± 1.28 mmHg and 7.96 ± 2.01, respectively. There was a significant difference in IMP between the SCI and sham groups (P < 0.05, F = 7.18, df = 1). Additionally, there was a significant difference between different time points in the IMP group (P < 0.05, F = 10.56, df = 18). There was no significant interaction between group and time point (P > 0.05, F = 1.19, df = 18). Sidak’s multiple comparisons test results showed that the difference between sham and SCI group was statistically significant at 42 hours (P < 0.05; Figure 4A).
Figure 4.
Dynamic changes in IMP in the first 72 hours post-SCI.
(A) Comparison of IMP between the sham (n = 8) and SCI groups (n = 8) by two-way repeated measures analysis of variance. The white circle represents the sham group, and the black square represents the SCI group. The IMP was always higher in the SCI group than in the sham group. The difference between the sham and SCI groups was statistically significant at 42 hours (*P < 0.05). (B) Comparison of IMP at different stages after SCI by linear regression analysis. The blue regression line indicates the IMP trend at stage I (P < 0.05), the red indicates the IMP trend at stage II (P < 0.05), and the orange indicates the IMP trend at stage III (P < 0.05). (C) Comparison of slopes (mmHg/h) between different stages. *P < 0.05 (mean ± SD, n = 8, one-way analysis of variance followed by Tukey’s post hoc test). IMP: Intramedullary pressure; SCI: spinal cord injury; h: hour(s).
IMP rapidly increased during the first 7 hours and then steadily increased during hours 8–38. Although IMP fluctuated during hours 39–72, overall it exhibited a downward trend. Three stages: I (steep rise), II (steady rise), and III (descending) were divided according to the changes in IMP in the SCI group (Figure 4B). The trends of each stage were described by linear regression analysis (Table 1), and there was a significant difference in slopes between stages (P < 0.05, F = 32.40, df = 2; Figure 4C).
Table 1.
Results of linear regression analysis in the SCI group
| Stage | Linear regression equation | r | Slope (mmHg/h) | P |
|---|---|---|---|---|
| I (1–7 h after SCI) | Y = 0.760 × X + 2.288 | 0.977 | 0.76 ± 0.12 | < 0.05 |
| II (8–38 h after SCI) | Y = 0.067 × X + 6.875 | 0.640 | 0.067 ± 0.018 | < 0.05 |
| III (39–72 h after SCI) | Y = –0.058 × X + 12.720 | –0.610 | –0.058 ± 0.021 | < 0.05 |
SCI: Spinal cord injury; h: hour(s).
Effect of SCI on neurologic outcome
The rabbits in the sham group showed no neurologic deficits, and their neurologic scores were 5.0 ± 0 after IMP monitoring. The Tarlov scores were 0 in the SCI group.
Effect of probe insertion on water content of the spinal cord
The spinal cord water content at 1 and 10 hours after probe insertion was 66.35 ± 1.07% and 67.07 ± 1.38%, respectively, and 68.13 ± 3.01% for intact animals. There was no significant difference among the three groups (P > 0.05).
Effect of SCI on blood volume of the spinal cord
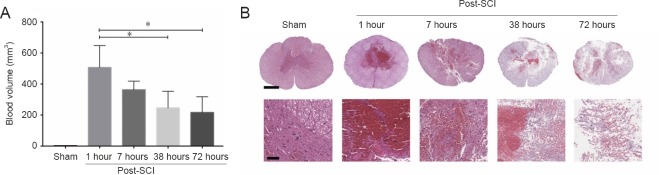
At 1 hour post-SCI, blood volume was 507.30 ± 140.90 mm³ (Figure 4). Severe hemorrhage was present within the injury epicenter only, predominantly within the gray matter, with substantial tissue disruption apparent. By 7 hours post-SCI, the injury area was enlarged, with a blood volume of 363.6 ± 54.93 mm³. By 38 hours post-SCI, diffuse hemorrhage (246.60 ± 107.00) was still apparent within the injury epicenter but was more reduced than at 1 hour (P < 0.05). The loss of tissue architecture was pronounced. By 72 hours post-SCI, diffuse hemorrhage (217.10 ± 100.50) had reduced within the injury epicenter compared with that at 1 hour (P < 0.05), while the loss of tissue architecture peaked. During the 72 hours post-SCI, spinal cord hemorrhage was most severe at 1 hour and then the blood volume gradually decreased. The blood volume in the sham group was 2.75 ± 0.40 mm3.
Effect of SCI on AQP4 immunoreactivity in the spinal cord
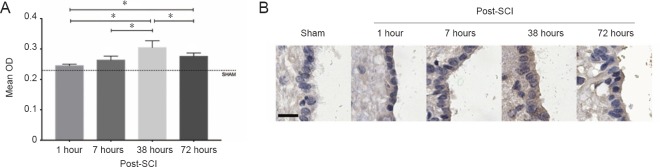
Sham sections of spinal cord showed faint AQP4 immunoreactivity in the ependymal cells of the central canal with the mean optical density of 0.23 ± 0.004 (Figure 5). Following injury, a slight increase was observed at 1 hour (0.25 ± 0.01). AQP4 immunoreactivity was significantly higher than sham at 7 hours (0.26 ± 0.01, P < 0.05) and peaked at 38 hours (0.30 ± 0.02, P < 0.05). By 72 hours, AQP4 immunoreactivity was slightly decreased (0.28 ± 0.01) but still higher than sham (0.23 ± 0.01, P < 0.05).
Figure 5.
Hematoxylin and eosin staining of blood volume (mm3) and morphological changes at different time points post-SCI.
(A) Comparison of blood volume (mm3) between the different groups. *P < 0.05 (mean ± SD, n =4, one-way analysis of variance followed by Tukey’s post hoc test). (B) Stained sections scanned by HistoFAXS 3.0 demonstrated severe hemorrhage in stage I. Higher magnification images clearly demonstrate the difference between time points. Scale bars: 1 mm (upper); 100 μm (lower). SCI: Spinal cord injury.
Effect of SCI on albumin immunoreactivity in the spinal cord
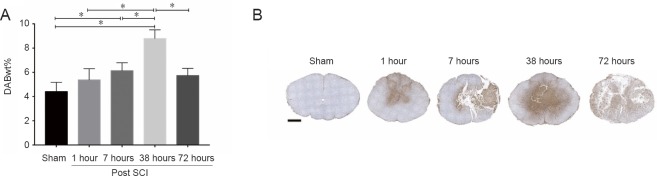
Sham sections showed minimal albumin immunoreactivity with a DABwt% by color deconvolution of 4.41 ± 0.76 (Figure 6). At 1 and 72 hours post-SCI, albumin immunoreactivity was 5.37 ± 0.92 and 5.74 ± 0.60, respectively. There was no significant difference, although the albumin immunoreactivity was higher than that of the sham group (P = 0.59, P = 0.21, respectively). Albumin immunoreactivity at both 7 and 38 hours post-SCI was significantly higher than sham (7 hours: DABwt% = 6.13 ± 0.65, P < 0.05; 38 hours: DABwt% = 8.79 ± 0.72, P < 0.05). Within 72 hours post-SCI, albumin immunoreactivity first increased and then decreased, peaking at 38 hours (Figure 7).
Figure 6.
Comparison of AQP4 immunoreactivity at different time points after SCI.
(A) Comparison of the mean OD of AQP4 staining within the central canal region of the spinal cord between different groups. *P < 0.05 (mean ± SD, n =4, one-way analysis of variance followed by Tukey’s post hoc test). (B) Anti-AQP4 immunohistochemistry sections at different time points scanned by HistoFAXS 3.0 demonstrated high AQP4 immunoreactivity at 38 hours post-SCI. Scale bar: 20 μm. SCI: Spinal cord injury; AQP4: aquaporin 4; OD: optical density.
Figure 7.
Comparison of albumin immunoreactivity at different time points after SCI.
(A) Color deconvolution for the albumin immunoreactivity. DABwt% values obtained represent an estimate of the amount of 3,3′-diaminobenzidine (DAB and thus antigen) on the original tissue section. Albumin immunoreactivity at 38 hours post-SCI was significantly higher than at other time points. *P < 0.05 (mean ± SD, n =4, one-way analysis of variance followed by Tukey’s post hoc test). (B) Anti-albumin immunohistochemistry sections at different time points scanned by HistoFAXS 3.0 demonstrated high albumin immunoreactivity at 38 hours post-SCI. Scale bar: 1 mm. SCI: Spinal cord injury.
Discussion
Preventing or reducing secondary injury has always been an important strategy for the treatment of SCI (Zhang et al., 2018). Increased IMP after SCI may cause more severe tissue damage and dysfunction (Batchelor et al., 2011). Thus, monitoring IMP has important clinical significance. Currently, there are few studies of IMP after SCI, and there was an urgent need to explore the dynamic changes of IMP after SCI. In this study, IMP was monitored in real-time for 72 hours in conscious rabbits using telemetry systems. Our results found that the dynamic changes were divided into three stages: IMP rapidly increased before 7 hours post-SCI, then went on to rise slowly during 8–38 hours, and then decreased during 39–72 hours. Spinal cord decompression during a steep rise may be more effective than after that stage in protecting surviving nerves after SCI.
Safety and advantages of telemetry for IMP measurement
An important feature of the telemetry system is the recording of physiological signals, such as arterial blood pressure and IMP, over extended periods of time in unstressed animals (i.e., unhindered by anesthesia or restraint). The batteries that power the telemeter are recharged noninvasively in vivo, using inductive power transfer. Consequently, the duration of experimental protocols is not limited by battery life. This new technology provides absolute values and the opportunity to examine the physiological signal changes in the progression of disease.
Under physiological conditions, subarachnoid pressure is similar to brain parenchymal pressure, and this measurement is routinely used to estimate intracranial pressure. The under-estimation of IMP using subarachnoid pressure recordings is a well-recognized phenomenon, especially during episodes of high intracranial pressure (Ostrup et al., 1987). The pressure in the subdural space is normal in 22.2% of SCI patients (Werndle et al., 2014). SCI leads to the acute rise of IMP before the spinal cord is constrained by the dural lining. (Khaing et al., 2017). Jones et al. (2012) observed the obliteration of the subarachnoid space in pigs with severe traumatic SCI within 10 minutes to 5 hours, whereas the obliteration of the subarachnoid space was seen in only 50% of the moderately injured animals. Only when the spinal cord is swollen, as caused by severe contusion and filling of the subarachnoid space, does the subarachnoid pressure approximate the parenchymal pressure of the spinal cord. Thus, the pressure in the subarachnoid space cannot reflect the real IMP. In this study, a pressure probe was inserted along the median dorsal aspect of the spinal cord (posterior mid-canal with fewer fiber bundles) to minimize the blunt trauma during implantation and to measure IMP, which can be a more accurate method. No functional abnormalities were observed between normal animals (probe implantation without SCI) and intact animals (without implantation and SCI) placed in same cage.
Leonard et al. (2013) found the intrathecal pressure of sham-operated rats immediately after the deflation of a balloon was lower (0.65 ± 1.61 mmHg). The pressure slightly increased and then stabilized during the 5-hour monitoring period, reaching 3.75 ± 1.26 mmHg at the conclusion of the study. A similar phenomenon was observed in our sham group: IMP continued to rise during the first 10 hours, reaching steady-state after 11 hours, with little change until the end of the study.
The probe insertion in this study was an invasive procedure and may increase the chance of hemorrhage, as suggested by the small drop in blood volume seen in the sham group. To detect whether the first increase in IMP was caused by implantation of the probe, the water content of the spinal cord was measured 1 and 10 hours after probe implantation in intact rabbits. The results showed no significant differences between these three groups, indicating that probe implantation may not be the dominant factor contributing to the increase in the first 10 hours.
There are different fine structures in the spinal cord parenchymal tissue, such as H-shaped (butterfly-type) gray matter with arranged nerve cell bodies within the internal area of the cord and peripheral white matter containing sensory and motor axons. Probe insertion from different angles may influence different cord structure, and it is not clear whether different tissue structures affect IMP. The probe angle can be fixed under animal anesthesia but cannot be easily controlled in awake animals. To observe whether it may interfere with IMP and to exclude this interference factor, three angles were set and the IMP monitored for 1 hour in anesthetized rabbits. The results showed that the angles had little effect on the IMP, which indicates that although the fine structures in spinal cord are different, IMP is similar in the same segment. The angle of probe cannot control when the IMP monitoring in conscious animal, but we can ignore this factor.
It has been reported that a normal IMP is 2.7 ± 0.5 mmHg (Khaing et al., 2017) or 6.88 ± 1.67 mmHg (Dong et al., 2016) in rats, 2.59 ± 1.01 mmHg in rabbits (Leonard et al., 2015), 8 ± 3 mmHg in mice (Saadoun et al., 2008), and 30 ± 12 mmHg in dogs (Iida and Tachibana, 1995). Most of the previous studies on IMP have only investigated anesthetized animals and provided information at only a single time point or a single time window (hours or minutes). In the current study, normal IMP in anesthetized rabbits (three angles) was similar to previous studies, and the differences could be attributed to different species and different measurement methods. However, normal IMP measured in conscious rabbits (sham group) was higher than anesthetized rabbits and also at 5 hours post-SCI. This may be because puncturing the dural sac during the probe implantation reduced the restraining effect on the spinal cord, and a small amount of cerebrospinal fluid outflowed, resulting in decompression. The effects caused by surgery were present in both anesthetized and conscious rabbits, but the latter had a longer recovery time of several hours, over which IMP reached a stable state.
Additionally, the effect of anesthesia on blood pressure cannot be ignored. Hypotension is one of the most common events in surgery (Soo et al., 2011; McCann and Schouten, 2014; Vachon et al., 2014). During anesthesia, sympathetic nerve activity is reduced, cardiac autonomic regulation is attenuated (Dorantes-Mendez et al., 2012), and hemodynamic changes occur. Anesthesia may have an indirect effect on IMP by affecting blood pressure in animals (spinal cord perfusion pressure = mean arterial pressure − IMP) (Kwon et al., 2009), which means the results from telemetry systems could be more accurate and reliable.
Dynamic changes in IMP in rabbits after SCI
Stage I (steep rise): IMP sharply increased during the 7 hours post-SCI. Several studies (Soubeyrand et al., 2012; Dong et al., 2016) have shown that intramedullary hemorrhage after SCI reaches its peak within hours, and in this study, blood volume at 1 and 7 hours post-SCI was greater than that at other time points. Therefore, intramedullary hemorrhage could be one of major causes of increased IMP during early SCI. Meanwhile, the cerebrospinal fluid compensatory mechanism may contribute to the maintenance of normal IMP (Chen et al., 2017), and surgical intervention such as that of the sham group may also play a role. With the worsening of a spinal cord hemorrhage and failure of the cerebrospinal fluid compensation mechanism, IMP sharply increased within 7 hours post-SCI.
Stage II (steady rise): At 7 hours post-SCI, IMP steadily increased until approximately 38 hours. Very-high-resolution ultrasound imaging showed that a hyperechoic lesion appeared immediately after SCI in the parenchyma and extended during the next 24 hours (Soubeyrand et al., 2014). Blood spinal cord barrier permeability peaked at 24 hours in mice with SCI (Badner et al., 2016) and lasted for a month in rats with SCI (Figley et al., 2014). Albumin is a plasma protein that is usually contained within the vasculature. When the blood-spinal cord barrier is broken, albumin enters spinal cord tissue, indicating that the blood spinal cord barrier is disrupted (Leonard and Vink, 2013). According to our results, albumin and AQP4 immunoreactivity were high at 38 hours post-SCI, revealing that the blood spinal cord barrier was destroyed by mechanical damage in this stage. This leads to inflammation, disruption of the microenvironment, and permeability changes in the vascular system. Edema, hemorrhage, and other factors after SCI lead to IMP that is higher than sham and that rises steadily. Surgical decompression within 7 hours post-injury (before stage II) could be the most efficacious.
Stage III (descending): At 38 hours post-SCI, although IMP fluctuated, it had an overall downward trend and on average was higher than during Stage II. Pan et al. (1999) found that blood spinal cord barrier permeability continued to increase within 48 hours post-SCI, reaching the first peak at 48 hours. Edema of the spinal cord occurs in early SCI and is severe at 2–3 days after SCI (Chavanne et al., 2011; Leonard et al., 2013). This is an important factor affecting IMP, especially in Stages II and III. Our results showed that blood volume, albumin, and AQP4 immunoreactivity were reduced during this period. This indicates that hemorrhage and edema of the spinal cord can gradually be absorbed and the IMP decreased.
At present, the effects of IMP on secondary injury after SCI are controversial. Increased IMP could cause more severe tissue damage and dysfunction after SCI (Saadoun et al., 2008), and tissue damage is significantly reduced when IMP decreases (Horn et al., 2008). However, we are not sure that IMP is the main factor, and other various unknown factors could be involved. Monitoring IMP has important significance in clinical settings, such as ensuring adequate blood perfusion of the spinal cord and guiding the timing and effect of surgical decompression after SCI. Because of delays caused by the delivery of patients to hospitals, various examinations, diagnosis and other factors, spinal cord decompression (such as surgical decompression and medication) cannot be performed immediately after SCI. It is of urgency to explore the time window of spinal cord decompression to achieve decompression within the optimal time to obtain the best effects. This study shows that IMP rises rapidly within the first 7 hours post-SCI, which may aggravate the secondary injury. Therefore, applying decompression before 7 hours post-SCI may effectively protect the spared nerve tissue and reduce the degree of secondary damage as much as possible, but this hypothesis needs the support of a large number of studies. The changes in IMP described in this study will support further exploration of the pathophysiological changes of SCI and provide guidance for other decompression methods following SCI.
Study limitations
Direct insertion of a pressure probe into the spinal cord may cause slight injury. Further research is needed to develop non-invasive methods in the future. IMP changes were measured only in the injury epicenter in this study, which does not involve the surrounding segments; including the surrounding segments could be an important future research direction. IMP was monitored here for only for 72 hours, and ideally this needs to be observed for 1 week or a longer after SCI. The pathological factors affecting IMP were the main focus here, while the influence of IMP on various pathological processes after SCI still needs further exploration. Additionally, IMP data in this study were drawn from the clip compression SCI model with only one injury level. In the future, the IMP should be investigated in SCI models with different injury mechanisms and severity.
Conclusions
IMP was measured in anesthetized and conscious rabbits using telemetry systems. The IMP after SCI was always higher than that of the sham group over 72 hours, and its dynamic changes were divided into three stages: I, steep rise (1–7 hours); II, steady rise (8–38 hours); and III, descending (39–72 hours). Severe spinal cord hemorrhage is the main reason for the sharp increase in IMP in early SCI, while edema and disruption of the blood spinal cord barrier are important factors in stages II and III. The current study may be used to guide the decompression of spinal cord in SCI patients.
Additional file: Open peer review report 1 (93.7KB, pdf) .
Acknowledgments:
Thank all the other members from the Department of Spinal and Neural Function Reconstruction, Beijing Bo’ai Hospital, China and Ying-Li Jing and Fan Bai from the Institute of Rehabilitation Science of China, China Rehabilitation Research Center, for their kind offer to guide the operation and the use of equipment.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81272164 (to JJL); the Special Fund for Basic Scientific Research of Central Public Research Institutes in China, No. 2016CZ-4 (to JJL), 2018CZ-1 (to JJL); the Beijing Institute for Brain Disorders in China, No. 0000-100031 (to JJL); the Basic Scientific Research Foundation of China Rehabilitation Research Center, No. 2017ZX-22, 2017ZX-20 (to JJL). The conception, design, execution, and analysis of experiments, as well as the preparation of and decision to publish this manuscript, were made independent of any funding organization.
Institutional review board statement: All the animal procedures were approved by Experimental Animal Welfare Committee (AEEI-2018-008) of Capital Medical University, China on January 8, 2018. All experimental procedures described here were in accordance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Kyoung-Tae Kim, Kyungpook National University, Republic of Korea.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81272164 (to JJL); the Special Fund for Basic Scientific Research of Central Public Research Institutes in China, No. 2016CZ-4 (to JJL), 2018CZ-1 (to JJL); the Beijing Institute for Brain Disorders in China, No. 0000-100031 (to JJL); the Basic Scientific Research Foundation of China Rehabilitation Research Center, No. 2017ZX-22, 2017ZX-20 (to JJL).
P-Reviewer: Kim KT; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Baba H, Tanoue Y, Maeda T, Kobayashi M, Oda S, Tominaga R. Protective effects of cold spinoplegia with fasudil against ischemic spinal cord injury in rabbits. J Vasc Surg. 2010;51:445–452. doi: 10.1016/j.jvs.2009.10.081. [DOI] [PubMed] [Google Scholar]
- 2.Badner A, Vawda R, Laliberte A, Hong J, Mikhail M, Jose A, Dragas R, Fehlings M. Early intravenous delivery of human brain stromal cells modulates systemic inflammation and leads to vasoprotection in traumatic spinal cord injury. Stem Cells Transl Med. 2016;5:991–1003. doi: 10.5966/sctm.2015-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batchelor PE, Kerr NF, Gatt AM, Cox SF, Ghasem-Zadeh A, Wills TE, Sidon TK, Howells DW. Intracanal pressure in compressive spinal cord injury: reduction with hypothermia. J Neurotrauma. 2011;28:809–820. doi: 10.1089/neu.2010.1622. [DOI] [PubMed] [Google Scholar]
- 4.Bazett-Jones DM, Tylinksi T, Krstic J, Stromquist A, Sparks J. Peak hip muscle torque measurements are influenced by sagittal plane hip position. Int J Sports Phys Ther. 2017;12:535–542. [PMC free article] [PubMed] [Google Scholar]
- 5.Borgens RB, Liu-Snyder P. Understanding secondary injury. Q Rev Biol. 2012;87:89–127. doi: 10.1086/665457. [DOI] [PubMed] [Google Scholar]
- 6.Chavanne A, Pettigrew DB, Holtz JR, Dollin N, Kuntz CT. Spinal cord intramedullary pressure in cervical kyphotic deformity: a cadaveric study. Spine (Phila Pa 1976) 2011;36:1619–1626. doi: 10.1097/BRS.0b013e3181fc17b0. [DOI] [PubMed] [Google Scholar]
- 7.Chen M, Yan Q, Sun J, Jin G, Qin M. Investigating the relationship between cerebrospinal fluid and magnetic induction phase shift in rabbit intracerebral hematoma expansion monitoring by MRI. Sci Rep. 2017;7:11186. doi: 10.1038/s41598-017-11107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S, Gallagher MJ, Papadopoulos MC, Saadoun S. Non-linear dynamical analysis of intraspinal pressure signal predicts outcome after spinal cord injury. Front Neurol. 2018;9:493. doi: 10.3389/fneur.2018.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, He Y, DeVivo MJ. Changing demographics and injury profile of new traumatic spinal cord injuries in the United States, 1972-2014. Arch Phys Med Rehabil. 2016;97:1610–1619. doi: 10.1016/j.apmr.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Dakson A, Brandman D, Thibault-Halman G, Christie SD. Optimization of the mean arterial pressure and timing of surgical decompression in traumatic spinal cord injury: a retrospective study. Spinal Cord. 2017;55:1033–1038. doi: 10.1038/sc.2017.52. [DOI] [PubMed] [Google Scholar]
- 11.Dong X, Yang D, Li J, Liu C, Yang M, Du L, Gu R, Hu A, Zhang H. Intramedullary pressure changes in rats after spinal cord injury. Spinal Cord. 2016;54:947–950. doi: 10.1038/sc.2016.35. [DOI] [PubMed] [Google Scholar]
- 12.Dorantes-Mendez G, Aletti F, Toschi N, Guerrisi M, Coniglione F, Dauri M, Baselli G, Signorini MG, Cerutti S, Ferrario M. Effects of propofol anesthesia induction on the relationship between arterial blood pressure and heart rate. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:2835–2838. doi: 10.1109/EMBC.2012.6346554. [DOI] [PubMed] [Google Scholar]
- 13.Fehlings MG, Tator CH. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord njury. Exp Neurol. 1995;132:220–228. doi: 10.1016/0014-4886(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 14.Figley SA, Khosravi R, Legasto JM, Tseng YF, Fehlings MG. Characterization of vascular disruption and blood-spinal cord barrier permeability following traumatic spinal cord injury. J Neurotrauma. 2014;31:541–552. doi: 10.1089/neu.2013.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furlan JC, Craven BC, Massicotte EM, Fehlings MG. Early versus delayed surgical decompression of spinal cord after traumatic cervical spinal cord injury: a cost-utility analysis. World Neurosurg. 2016;8:166–174. doi: 10.1016/j.wneu.2015.12.072. [DOI] [PubMed] [Google Scholar]
- 16.Guild SJ, McBryde FD, Malpas SC. Recording of intracranial pressure in conscious rats via telemetry. J Appl Physio (1985) 2015;119:576–581. doi: 10.1152/japplphysiol.00165.2015. [DOI] [PubMed] [Google Scholar]
- 17.Horn EM, Theodore N, Assina R, Spetzler RF, Sonntag VK, Preul MC. The effects of intrathecal hypotension on tissue perfusion and pathophysiological outcome after acute spinal cord injury. Neurosurg Focus. 2008;25:E12. doi: 10.3171/FOC.2008.25.11.E12. [DOI] [PubMed] [Google Scholar]
- 18.Hou S, Blesch A, Lu P. A radio-telemetric system to monitor cardiovascular function in rats with spinal cord transection and embryonic neural stem cell grafts. J Vis Exp. 2014:e51914. doi: 10.3791/51914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu AM, Li JJ, Sun W, Yang DG, Yang ML, Du LJ, Gu R, Gao F, Li J, Chu HY, Zhang X, Gao LJ. Myelotomy reduces spinal cord edema and inhibits aquaporin-4 and aquaporin-9 expression in rats with spinal cord injury. Spinal Cord. 2015;53:98–102. doi: 10.1038/sc.2014.209. [DOI] [PubMed] [Google Scholar]
- 20.Iida H, Tachibana S. Spinal cord intramedullary pressure: direct cord traction test. Neurol Med Chir (Tokyo) 1995;35:75–77. doi: 10.2176/nmc.35.75. [DOI] [PubMed] [Google Scholar]
- 21.Jones CF, Cripton PA, Kwon BK. Gross morphological changes of the spinal cord immediately after surgical decompression in a large animal model of traumatic spinal cord injury. Spine (Phila Pa 1976) 2012;37:890–899. doi: 10.1097/BRS.0b013e3182553d1d. [DOI] [PubMed] [Google Scholar]
- 22.Jones CF, Lee JH, Kwon BK, Cripton PA. Development of a large-animal model to measure dynamic cerebrospinal fluid pressure during spinal cord injury: Laboratory investigation. J Neurosurg Spine. 2012;16:624–635. doi: 10.3171/2012.3.SPINE11970. [DOI] [PubMed] [Google Scholar]
- 23.Khaing ZZ, Cates LN, Fischedick AE, McClintic AM, Mourad PD, Hofstetter CP. Temporal and spatial evolution of raised intraspinal pressure after traumatic spinal cord injury. J Neurotrauma. 2017;34:645–651. doi: 10.1089/neu.2016.4490. [DOI] [PubMed] [Google Scholar]
- 24.Koeners MP, Ow C, Russell DM, Evans RG, Malpas SC. Prolonged and continuous measurement of kidney oxygenation in conscious rats. Methods Mol Biol. 2016;1397:93–111. doi: 10.1007/978-1-4939-3353-2_9. [DOI] [PubMed] [Google Scholar]
- 25.Kreinest M, Ludes L, Biglari B, Kuffer M, Turk A, Grutzner PA, Matschke S. Influence of previous comorbidities and common complications on motor function after early surgical treatment of patients with traumatic spinal cord injury. J Neurotrauma. 2016;33:2175–2180. doi: 10.1089/neu.2016.4416. [DOI] [PubMed] [Google Scholar]
- 26.Kwon BK, Curt A, Belanger LM, Bernardo A, Chan D, Markez JA, Gorelik S, Slobogean GP, Umedaly H, Giffin M, Nikolakis MA, Street J, Boyd MC, Paquette S, Fisher CG, Dvorak MF. Intrathecal pressure monitoring and cerebrospinal fluid drainage in acute spinal cord injury: a prospective randomized trial. J Neurosurg Spine. 2009;10:181–193. doi: 10.3171/2008.10.SPINE08217. [DOI] [PubMed] [Google Scholar]
- 27.Lee BB, Cripps RA, Fitzharris M, Wing PC. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. 2014;52:110–116. doi: 10.1038/sc.2012.158. [DOI] [PubMed] [Google Scholar]
- 28.Leonard AV, Vink R. The effect of an NK1 receptor antagonist on blood spinal cord barrier permeability following balloon compression-induced spinal cord injury. Acta Neurochir Suppl. 2013;118:303–306. doi: 10.1007/978-3-7091-1434-6_59. [DOI] [PubMed] [Google Scholar]
- 29.Leonard AV, Thornton E, Vink R. Substance P as a mediator of neurogenic inflammation after balloon compression induced spinal cord injury. J Neurotrauma. 2013;30:1812–1823. doi: 10.1089/neu.2013.2993. [DOI] [PubMed] [Google Scholar]
- 30.Leonard AV, Thornton E, Vink R. The relative contribution of edema and hemorrhage to raised intrathecal pressure after traumatic spinal cord injury. J Neurotrauma. 2015;32:397–402. doi: 10.1089/neu.2014.3543. [DOI] [PubMed] [Google Scholar]
- 31.Liu C, Yang D, Li J, Li D, Yang M, Sun W, Meng Q, Zhang W, Cai C, Du L, Li J, Gao F, Gu R, Feng Y, Dong X, Miao Q, Yang X, Zuo Z. Dynamic diffusion tensor imaging of spinal cord contusion: A canine model. J Neurosci Res. 2018;96:1093–1103. doi: 10.1002/jnr.24222. [DOI] [PubMed] [Google Scholar]
- 32.Liu CB, Yang DG, Meng QR, Li DP, Yang ML, Sun W, Zhang WH, Cai C, Du LJ, Li J, Gao F, Yu Y, Zhang X, Zuo ZT, Li JJ. Dynamic correlation of diffusion tensor imaging and neurological function scores in beagles with spinal cord injury. Neural Regen Res. 2018;13:877–886. doi: 10.4103/1673-5374.232485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Shi CG, Wang XW, Chen HJ, Wang C, Cao P, Gao R, Ren XJ, Luo ZJ, Wang B, Xu JG, Tian JW, Yuan W. Timing of surgical decompression for traumatic cervical spinal cord injury. Int Orthop. 2015;39:2457–2463. doi: 10.1007/s00264-014-2652-z. [DOI] [PubMed] [Google Scholar]
- 34.Martirosyan NL, Kalani MY, Bichard WD, Baaj AA, Gonzalez LF, Preul MC, Theodore N. Cerebrospinal fluid drainage and induced hypertension improve spinal cord perfusion after acute spinal cord injury in pigs. Neurosurgery. 2015;76:461–469. doi: 10.1227/NEU.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 35.Matsushita A, Maeda T, Mori E, Yuge I, Kawano O, Ueta T, Shiba K. Can the acute magnetic resonance imaging features reflect neurologic prognosis in patients with cervical spinal cord injury? Spine J. 2017;17:1319–1324. doi: 10.1016/j.spinee.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 36.McCann ME, Schouten AN. Beyond survival; influences of blood pressure, cerebral perfusion and anesthesia on neurodevelopment. Paediatr Anaesth. 2014;24:68–73. doi: 10.1111/pan.12310. [DOI] [PubMed] [Google Scholar]
- 37.Miyanji F, Furlan JC, Aarabi B, Arnold PM, Fehlings MG. Acute cervical traumatic spinal cord injury: MR imaging findings correlated with neurologic outcome--prospective study with 100 consecutive patients. Radiology. 2007;243:820–827. doi: 10.1148/radiol.2433060583. [DOI] [PubMed] [Google Scholar]
- 38.Noussitou FL, Gorgas D, Rohrbach H, Henke D, Howard J, Forterre F. Assessment of intramedullary spinal pressure in small breed dogs with thoracolumbar disk extrusion undergoing hemilaminectomy. Vet Surg. 2015;44:944–948. doi: 10.1111/vsu.12399. [DOI] [PubMed] [Google Scholar]
- 39.Ostrup RC, Luerssen TG, Marshall LF, Zornow MH. Continuous monitoring of intracranial pressure with a miniaturized fiberoptic device. J Neurosurg. 1987;67:206–209. doi: 10.3171/jns.1987.67.2.0206. [DOI] [PubMed] [Google Scholar]
- 40.Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp (Wars) 2011;71:281–299. doi: 10.55782/ane-2011-1848. [DOI] [PubMed] [Google Scholar]
- 41.Pan W, Kastin AJ, Bell RL, Olson RD. Upregulation of tumor necrosis factor alpha transport across the blood-brain barrier after acute compressive spinal cord injury. J Neurosci. 1999;19:3649–3655. doi: 10.1523/JNEUROSCI.19-09-03649.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phang I, Zoumprouli A, Papadopoulos MC, Saadoun S. Microdialysis to optimize cord perfusion and drug delivery in spinal cord injury. Ann Neurol. 2016;80:522–531. doi: 10.1002/ana.24750. [DOI] [PubMed] [Google Scholar]
- 43.Piazza M, Schuster J. Timing of surgery after spinal cord injury. Neurosurg Clin N AM. 2017;28:31–39. doi: 10.1016/j.nec.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 44.Renfu Q, Rongliang C, Mengxuan D, Liang Z, Jinwei X, Zongbao Y, Disheng Y. Anti-apoptotic signal transduction mechanism of electroacupuncture in acute spinal cord injury. Acupunct Med. 2014;32:463–471. doi: 10.1136/acupmed-2014-010526. [DOI] [PubMed] [Google Scholar]
- 45.Saadoun S, Bell BA, Verkman AS, Papadopoulos MC. Greatly improved neurological outcome after spinal cord compression injury in AQP4-deficient mice. Brain. 2008;131:1087–1098. doi: 10.1093/brain/awn014. [DOI] [PubMed] [Google Scholar]
- 46.Soo JC, Lacey S, Kluger R, Silbert BS. Defining intra-operative hypotension-a pilot comparison of blood pressure during sleep and general anaesthesia. Anaesthesia. 2011;66:354–360. doi: 10.1111/j.1365-2044.2011.06657.x. [DOI] [PubMed] [Google Scholar]
- 47.Soubeyrand M, Badner A, Vawda R, Chung YS, Fehlings MG. Very high resolution ultrasound imaging for real-time quantitative visualization of vascular disruption after spinal cord injury. J Neurotrauma. 2014;31:1767–1775. doi: 10.1089/neu.2013.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soubeyrand M, Laemmel E, Dubory A, Vicaut E, Court C, Duranteau J. Real-time and spatial quantification using contrast-enhanced ultrasonography of spinal cord perfusion during experimental spinal cord injury. Spine (Phila Pa 1976) 2012;37:1376–1382. doi: 10.1097/BRS.0b013e318269790f. [DOI] [PubMed] [Google Scholar]
- 49.Soubeyrand M, Laemmel E, Court C, Dubory A, Vicaut E, Duranteau J. Rat model of spinal cord injury preserving dura mater integrity and allowing measurements of cerebrospinal fluid pressure and spinal cord blood flow. Eur Spine J. 2013;22:1810–1819. doi: 10.1007/s00586-013-2744-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vachon C, Belanger MC, Burns PM. Evaluation of oscillometric and Doppler ultrasonic devices for blood pressure measurements in anesthetized and conscious dogs. Res Vet Sci. 2014;97:111–117. doi: 10.1016/j.rvsc.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Varghese F, Bukhari AB, Malhotra R, De A. IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One. 2014;9:e96801. doi: 10.1371/journal.pone.0096801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vink R, Young A, Bennett CJ, Hu X, Connor CO, Cernak I, Nimmo AJ. Neuropeptide release influences brain edema formation after diffuse traumatic brain injury. Acta Neurochir Suppl. 2003;86:257–260. doi: 10.1007/978-3-7091-0651-8_55. [DOI] [PubMed] [Google Scholar]
- 53.Werndle MC, Saadoun S, Phang I, Czosnyka M, Varsos GV, Czosnyka ZH, Smielewski P, Jamous A, Bell BA, Zoumprouli A, Papadopoulos MC. Monitoring of spinal cord perfusion pressure in acute spinal cord injury: initial findings of the injured spinal cord pressure evaluation study. Crit Care Med. 2014;42:646–655. doi: 10.1097/CCM.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 54.Yang DG, Li JJ, Gu R, Yang ML, Zhang X, Du LJ, Sun W, Gao F, Hu AM, Wu YY, He JG, Feng YT, Chu HY. Optimal time window of myelotomy in rats with acute traumatic spinal cord injury: a preliminary study. Spinal Cord. 2013;51:673–678. doi: 10.1038/sc.2013.56. [DOI] [PubMed] [Google Scholar]
- 55.Yang XX, Huang ZQ, Li ZH, Ren DF, Tang JG. Risk factors and the surgery affection of respiratory complication and its mortality after acute traumatic cervical spinal cord injury. Medicine (Baltimore) 2017;96:e7887. doi: 10.1097/MD.0000000000007887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Shen CL, Dong FL, Zhang RJ, Ge P. Correlation of cytokine levels in the peripheral blood within 24 hours after cervical spinal cord injury with the American Spinal Injury Association impairment scale: a comparative study. Zhongguo Zuzhi Gongcheng Yanjiu. 2018;22:3824–3830. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.