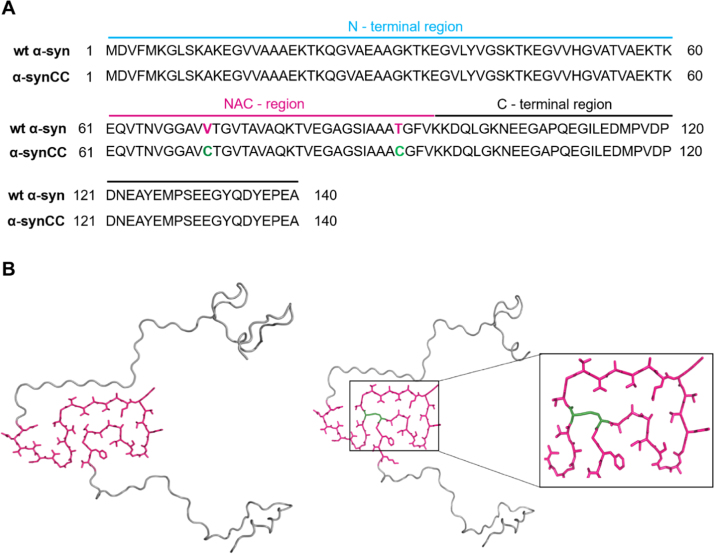
Fig. 1.
Primary sequence and three-dimensional structure of wt α-syn and its double cysteine mutant α-synCC. (A) Primary sequence alignment of wt α-syn and α-synCC. The amino acid sequences of wt α-syn (UniProt ID: P37840) and α-synCC are shown. The three distinct regions of α-syn are indicated. (B) Structural models of wt α-syn (left panel) and α-synCC (right panel) showing the NAC domain in purple. The models were generated in PyMol using the structure of pathogenic fibril of full-length human α-syn (PDB: 2N0A). Cys71 and Cys92 residues in α-synCC are shown in green.