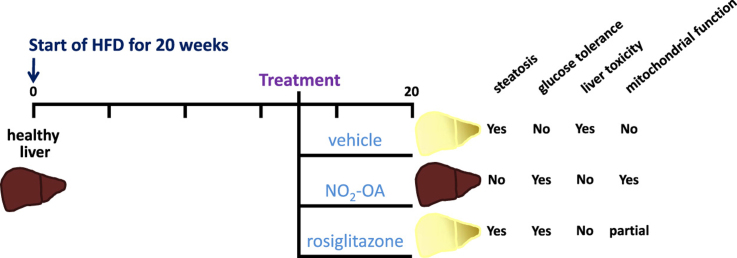
Abstract
Non-alcoholic fatty liver disease (NAFLD) is linked to obesity and insulin resistance and is the most prevalent chronic liver disease. During the development of obesity and NAFLD, mitochondria adapt to the increased lipid load in hepatocytes by increasing the rate of fatty acid oxidation. In concert with this, reactive species (RS) generation is increased, damaging hepatocytes and inducing inflammation. Hepatic mitochondrial dysfunction is central to the pathogenesis of NAFLD via undefined mechanisms. There are no FDA approved treatments for NAFLD other than weight loss and management of glucose tolerance. Electrophilic nitro-oleic acid (NO2-OA) displays anti-inflammatory and antioxidant signaling actions, thus mitochondrial dysfunction, RS production and inflammatory responses to NO2-OA and the insulin sensitizer rosiglitazone were evaluated in a murine model of insulin resistance and NAFLD. Mice on HFD for 20 wk displayed increased adiposity, insulin resistance and hepatic lipid accumulation (steatosis) compared to mice on normal chow (NC). The HFD mice had mitochondrial dysfunction characterized by lower hepatic mitochondrial complex I, IV and V activity compared to mice on NC. Treatment with NO2-OA or rosiglitazone for the last 42 days (out of 20 wk) abrogated HFD-mediated decreases in hepatic mitochondrial complex I, IV and V activity. Notably, NO2-OA treatment normalized hepatic triglyceride levels and significantly reversed hepatic steatosis. Despite the improved glucose tolerance observed upon rosiglitazone treatment, liver weight and hepatic triglycerides were significantly increased over vehicle-treated HFD mice. These observations support that the pleiotropic signaling actions of electrophilic fatty acids limit the complex hepatic and systemic pathogenic responses instigated by obesity, without the adverse effects of thiazolidinedione drugs such as rosiglitazone.
Graphical abstract
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most prevalent chronic liver disease. The most common risk factors associated with NAFLD are obesity, type 2 diabetes and dyslipidemia, with the prevalence of NAFLD occurring in 80–90% of obese adults [1], [2]. This disease is largely under-reported, considering that subjects with NAFLD are asymptomatic and the prevalence of obesity is still skyrocketing. The hallmark of NAFLD is neutral lipid accumulation, mainly in the form of intra-hepatic triglyceride (steatosis), in the absence of significant alcohol consumption. In general, hepatic steatosis is defined as intrahepatic fat > 5% of liver volume, a response that occurs when the balance shifts towards the uptake of free fatty acids (FFA) and de novo lipogenesis from the oxidation of FFA. The spectrum of disease starts with simple steatosis and is generally inert pathologically. However, a simple fatty liver in the presence of inflammation, defined as non-alcoholic steatohepatitis (NASH), leads to fibrosis, cirrhosis and eventually results in hepatocellular carcinoma, liver failure and death [3]. As waistlines continue to expand, NAFLD is now considered the next global epidemic, as it is associated with increased risk of metabolic syndrome, cardiovascular disease and cancer.
Besides lifestyle changes, there are no FDA-approved medications for the treatment of the more aggressive form of NAFLD, non-alcoholic steatohepatitis (NASH), defined as steatosis with inflammation with or without fibrosis. Lifestyle changes are the first and only accepted line of therapy at the moment, centering on a decrease in weight by improving diet, increasing physical activity and preventing sedentarity [4], [5], [6], [7]. Lifestyle changes have worked with limited success as the key for patients is to sustain this loss in body weight, which is often difficult to do. Therefore, drugs that limit hepatocyte lipotoxicity are absolutely needed. Understanding the pathophysiology of this multifactorial complex disease will help design therapeutics to address the unmet need in NAFLD and NASH.
Of all the drugs tested for NASH, the most promising therapeutic based on randomized controlled trials (RCT) may be thiazolidinediones (TZDs) [8]. The current guidelines for the treatment of NASH recommend TZDs [5]. Hepatic and whole-body insulin resistance is strongly associated with hepatic steatosis while improving insulin sensitivity, generally, has protective effects in NAFLD and NASH [9], [10], [11]. TZDs are potent insulin sensitizers that are agonists of the peroxisome proliferator-activated receptor-γ (PPARγ), although the exact mechanism of action is not completely understood. The two key findings from open label and RCTs with TZDs show decreases in steatosis and peripheral indices of liver injury such as alanine aminotransferase (ALT) [8]. One mechanism that may account for this response is that TZDs decrease circulating free fatty acids (FFA), which divert the FFA towards adipocytes instead of the liver, thereby limits the FFA load on the liver [12], [13]. As potent as TZDs are as insulin sensitizers, adverse side effects, such as increased incidence of cardiovascular events, congestive heart failure, edema, weight gain (due to increased fat mass) and certain cancers [13], [14], [15], [16], [17], severely compromise the use of this class of drugs. Additionally, long-term patient compliance is also negatively impacted by weight gain. More studies are warranted to determine whether long-term TZD use remains a viable treatment option. Better yet, a therapeutic agent that can target multiple comorbidities associated with this disease without unwanted side effects would be an advance.
Over the last decade, numerous studies have demonstrated that electrophilic nitro-fatty acids (NO2-FA) display beneficial metabolic and anti-inflammatory actions that inhibit reactive species generation and pro-inflammatory signaling pathways. The mechanism of action of NO2-FAs is hypothesized to be via post-translational modification of multiple transcriptional regulatory factors and pro-inflammatory enzymes. Preclinical models supporting these actions include allergic airway disease [18], atherosclerosis [19], diabetic kidney disease [20], [21], endotoxin-induced vascular inflammation and multi-organ injury [22], [23], hypertension [24], [25], insulin resistance [26] and pulmonary arterial hypertension (PAH) [27], [28]. Beyond these experimental animal models, multiple phase I clinical studies (NCT: 02127190, 02248051, 02460146, 02313064, 02547402) have now been successfully completed with 10-nitro-octadec-9-enoic acid (NO2-OA); and patients are currently being enrolled for phase II clinical trials for the treatment of focal segmental glomerulosclerosis and PAH [29].
Due to the fact that steatosis is multifactorial, a drug that targets multiple pathways could be a beneficial therapy. A therapeutic that can improve insulin sensitivity, while at the same time decreasing inflammation, oxidative stress and fibrosis, may be of benefit for treating NAFLD or NASH. The effects of NO2-OA include limiting inflammation, oxidative stress and fibrosis by suppressing downstream NF-κB signaling [30], [31], upregulating Nrf2 transactivation [32], inducing dedifferentiation of myofibroblasts [33], [34] and inhibiting the catalytic activity of soluble epoxide hydrolase [35], NADPH oxidase [36] and xanthine oxidase [37], [38]. The goal of this study was to assess whether NO2-OA attenuates high fat diet-induced obesity (DIO)-induced hepatic steatosis and insulin resistance. A model of DIO was used because the metabolic phenotype more closely resembles what is observed clinically and the metabolic actions of NO2-OA were compared to one of the TZDs recommended to treat NASH, rosiglitazone.
2. Methods
2.1. Mouse model
All animal studies were conducted under the approval of the University of Pittsburgh Institutional Animal Care and Use Committee (protocol 17019691). Male C57Bl/6j mice were purchased from Jackson Laboratory (Bar Harbor, ME). The diets were purchased from Research Diets Inc. (New Brunswick, NJ). Obesity was induced by the HFD (D12492, with 60% of the adjusted calories derived from fat) for 20 wk beginning at age 6–8 wk. Age-matched controls (n = 8) were maintained on LFD (D12450J, 10% of the adjusted calories derived from fat with 7% sucrose match with D12492). Diet and water were supplied ad libitum for 20 wk. Food intake and mouse weight were monitored twice per wk. At wk 14 of the HFD study, mice were anesthetized with isoflurane before Alzet osmotic pumps (Cupertino, CA) containing vehicle (polyethylene glycol/ethanol) (n = 8), 10-nitro-octadec-9-enoic acid (nitro-oleic acid, NO2-OA) (n = 8) or rosiglitazone (n = 6) were implanted subcutaneously in the back region, as previously described [38]. The osmotic mini pump delivered a concentration of 8 mg/kg/d. There was no difference in food intake after administration of the treatments on HFD (units are g food/day; vehicle= 2.7, NO2-OA = 2.8 and rosi = 2.7).
2.2. In vivo studies
Mice were fasted for 5 h and fasting blood glucose and glucose tolerance test (GTT) were performed as previously described [38]. Body weight and all tissue/organ weights were measured using a precision scale (Scout Pro). Body composition was assessed using EchoMRI model 100 H (EchoMRI).
2.3. Blood chemistry assays
Mice were fasted for 5 h for all blood collections. Terminal blood draw was collected by cardiac puncture during sacrifice under isoflurane anesthesia using heparinized syringes and was kept on ice until centrifugation at 3000 g for 30 min at 4 °C for plasma isolation. Total triglycerides and cholesterol concentrations were determined using enzymatic kits according to the manufacturer's instructions from Cayman Chemical (10010303) and Raichem (R80015), respectively. Nonesterified fatty acids were measured using the NEFA-HR kit (Wako Chemicals). Plasma levels of alanine aminotransferase (ALT) were determined spectrophotometrically using standard kits (Thermo Fisher Scientific, Waltham, MA), as described previously [39].
2.4. Hepatic triglycerides
Briefly, frozen liver samples (~150 mg) were thawed on ice and homogenized in a bullet blender (Next Advance) for 5 min in 50 mM phosphate buffer (pH 7.4) with butylated hydroxytoluene (0.16%) to a final concentration of 0.5 mg tissue/ml. The liver homogenate was spiked with 2.34 nmol internal standard glyceryl triheptadecanoate (Nu-Check Prep) and then the triglycerides were extracted using the Bligh and Dyer procedure [40]. The organic extracts were dried under nitrogen, reconstituted in ethyl acetate, diluted 80 × (in ethyl acetate) and the hepatic lipid profile and molecular species were determined by HPLC-ESI-MS/MS analysis as previously described [41], [42].
2.5. Mitochondrial function
Respiration was measured in isolated liver mitochondria utilizing a Clark-type oxygen electrode (Instech Inc) as previously described [43]. Briefly, mitochondria were suspended at 1 mg/ml in respiration buffer and state 4 respiration was initiated by the addition of succinate (1 mM). State 3 respiration was then initiated by the addition of ADP (20 mM) and RCR was calculated as the ratio of state 3 to state 4.
The activities of complexes I, IV and V were measured spectrophotometrically as previously described [44], [45], [46]. Citrate Synthase activity was measured using oxaloacetate and acetyl CoA as substrates, coupling the (a) citrate synthase generation of citrate and CoA with the (b) reaction of CoA with DTNB. The formation of DTNB-CoA was monitored spectrophotometrically at 412 nm. Diluted supernatant (10–30 μg) was equilibrated at 37 °C in a reaction mix containing 400 μM DTNB, 200 μM acetyl-CoA, 100 mM Tris pH 8.0% and 0.1% triton X-100. Reactions were initiated by the addition of 200 μM Oxaloacetate. The increase in absorbance at 412 nm was monitored for 10 min and the rate of DTNB conversion to DTNB-CoA was expressed as pmol/min/mg protein based on the extinction coefficient of 13,600 (mol/min/liter) for DTNB-CoA. Complex I activity was measured using NADH and ubiquinone as substrates and monitoring the rotenone sensitive decrease in absorbance of NADH at 340 nm. Diluted supernatant (10–30 μg) was equilibrated at 37 °C in a reaction mixture containing 25 mM KPO4 pH 7.2, 10 mM MgCl2, 2.5 mg/ml BSA, 1 mM KCN and 0.1 mM NADH. Reactions were initiated by 50 μM decylubiquinone and the decrease in absorbance at 340 nm was monitored for 10 min, after which rotenone was added to a final concentration of 10 μM and the reaction was monitored for an additional 10 min. The rotenone sensitive rate was expressed as pmol/min/mg protein based on the extinction coefficient of 6180 (mol/min/liter) for NADH. Complex IV activity was measured using reduced cytochrome c as the substrate and monitoring the oxidation of cytochrome c at 550 nm. Diluted supernatant (1–10 μg) was equilibrated to 30 °C in 10 mM KPO4 pH 7.0 and the reaction was initiated by the addition of 50 μM reduced cytochrome c. The decrease in absorbance at 550 nm was monitored for 3 min and the oxidation of cytochrome c was expressed as k/min/mg protein. Complex V activity monitored the rate of NADH oxidation in the presence of ADP at 340 nm. Amplex Red (Thermo) was used to determine the rate of hydrogen peroxide (H2O2) generation in freshly isolated mitochondria as previously described [47]. Briefly, the oxidation of Amplex Red (50 µM) to resorufin was monitored spectrophotometrically (excitation and emission wavelengths of 571 nm and 585 nm). Catalase (100 µM) was added to a subset of samples to ensure that the signal being detected was due to H2O2.
3. Results
3.1. Rosiglitazone treated mice have increased body weight and fat mass compared to NO2-OA
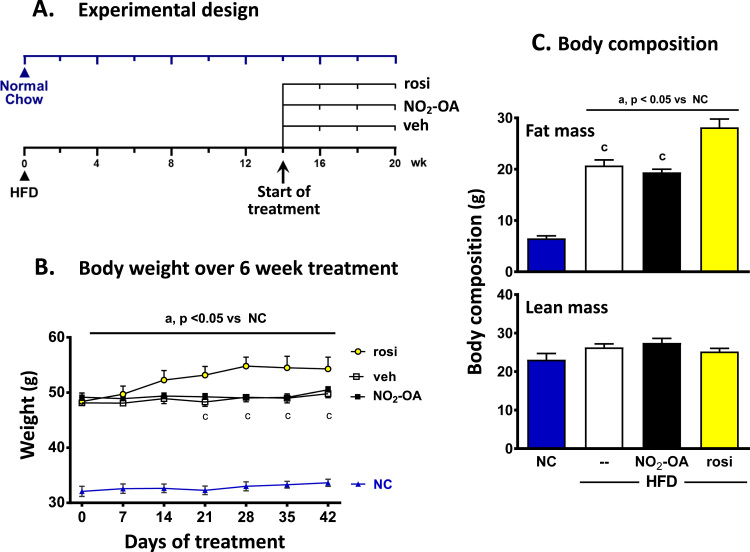
The mice were subjected to a HFD for a total of 20 wk with drug treatments starting at wk 14. The mice subjected to a HFD gained weight compared with age-matched mice on normal chow (NC). At wk 14, the HFD-fed mice were randomly assigned to a treatment group consisting of vehicle, NO2-OA or rosiglitazone (rosi) for the last 6 wk of the 20 wk study. The NC mice were treated with vehicle. In Fig. 1A, the day of treatment at time 0 refers to the initiation of the intervention (vehicle, NO2-OA or rosiglitazone) at wk 14. The HFD-fed mice treated with rosiglitazone gained significantly more weight than the vehicle or NO2-OA-treated mice starting at d 21 of drug administration. This weight difference persisted until the end of the 6 wk treatment (a total of 20 wk on HFD) in Fig. 1B. Similarly, rosiglitazone-treated mice had increased fat mass over vehicle- and NO2-OA-treated mice on the HFD at d 30 of treatment. There was no difference in body weight or adiposity between the HFD mice treated with NO2-OA or vehicle. As expected, the mice on the HFD had significantly greater adiposity compared to the age-matched NC mice treated with vehicle (Fig. 1C) and there was no difference in lean mass between the groups.
Fig. 1.
Rosiglitazone treated mice have increased weight gain and fat mass compared to NO2-OA or vehicle treated mice on the HFD. (A) Mice were subjected to a HFD or normal chow (NC) for a total of 20 weeks. At week 14 (initiation of treatment, day 0), the HFD-fed mice were randomly assigned to a treatment group consisting of vehicle, NO2-OA or rosiglitazone (rosi) for the last 6 week of the 20 week HFD study. Mice on NC were treated with vehicle. (B) The body weight was monitored over the 6 week treatment period. (C) The body composition of the treated mice was measured at day 30. All the data are the mean ± SEM. A two-way and one-way ANOVA was used for weight measurements and body composition, respectively. Significance was determined as: a, p<0.05 vs NC; c, p<0.05 vs rosi-treated HFD mice.
3.2. NO2-OA improves glucose tolerance
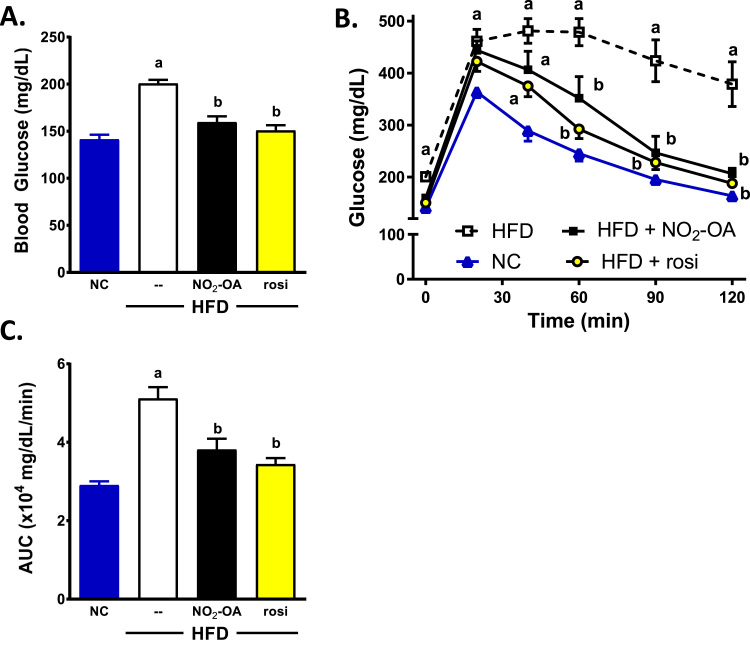
There was a significant increase in fasting blood glucose levels in the HFD mice treated with vehicle (open square) compared to NC (Fig. 2A; 200.6 ± 4.0 vs 141.3 ± 5.0, p < 0.0001). Additionally, the HFD mice had impaired glucose tolerance compared to NC mice treated with vehicle (Fig. 2B). Administration of NO2-OA (black squares) resulted in a significant decrease in fasting blood glucose levels and improved glucose tolerance compared to HFD mice treated with vehicle. The fasting glucose levels were similar for NO2-OA- and rosiglitazone-treated mice (159.5 ± 6.2 vs 150.7 ± 5.6, respectively). The area under the glucose response curves (AUC) for both NO2-OA and rosiglitazone were significantly decreased compared to HFD mice with vehicle (Fig. 2C). There was no statistical difference in fasting blood glucose or AUC between NO2-OA, rosiglitazone and age-matched NC treated with vehicle.
Fig. 2.
NO2-OA treatment lowers fasting blood glucose levels and improves glucose tolerance similar to Rosi treated HFD mice. On day 35 of treatment, all mice were fasted for 5 h and fasting blood glucose levels (A) were measured at time 0 of the glucose tolerance test (GTT). (B) The GTT consisted of blood glucose measurements at 20, 40, 60, 90 and 120 min following an intraperitoneal injection with 1.3 mg/kg glucose solution. (C) The area under the curve (AUC) during the GTT was calculated and plotted. All the data are the mean ± SEM. A two-way ANOVA was used for the GTT (B) and one-way ANOVA was used for fasting blood glucose levels (A) and area under the glucose response curves (C). Significance was determined as: a, p < 0.05 vs NC; b, p < 0.05 vs HFD mice treated with vehicle.
3.3. NO2-OA limits HFD-induced dyslipidemia
The mice on the HFD for 20 wk had increased cholesterol, NEFA and triglycerides (TG) compared to the age-matched mice on NC. Both NO2-OA and rosiglitazone treatment decreased circulating TG and there was a trend for a decrease in NEFA (although not significant) compared to HFD (Table 1). NEFA were measured as indices of free fatty acids (FFA). All three HFD treatment groups had a significant increase in circulating cholesterol levels compared to NC.
Table 1.
Plasma alanine aminotransferase (ALT), cholesterol, NEFA and triglyceride levels in NC or HFD-fed mice treated with vehicle, NO2–OA or rosiglitazone. Mice on the NC were treated with vehicle for the last 42 days out the 20 week study. The plasma used was from the terminal blood draw. Data are the mean ± SEM. One-way ANOVA was used and significance was determined as: a, p < 0.05 vs NC; b, p < 0.05 vs HFD mice treated with vehicle.
| Normal Chow (NC) |
High Fat Diet (HFD) |
|||
|---|---|---|---|---|
| Parameters | Vehicle | Vehicle | NO2-OA | Rosi |
| ALT (IU/L) | 121.5 ± 10.9 b | 337.5 ± 68.8 | 157.1 ± 47.0b | 102.0 ± 13.9 b |
| Cholesterol (mg/dL) | 91.2 ± 18.0 | 214.1 ± 10.7a | 217.7 ± 30.0a | 194.4 ± 14.0 a |
| NEFA (mEq/L) | 0.32 ± 0.031 | 0.54 ± 0.034a | 0.44 ± 0.028 | 0.42 ± 0.022 |
| Triglyceride (mg/dL) | 71.4 ± 5.1b | 128.9 ± 15.5 | 65.6 ± 8.8b | 65.5 ± 9.7b |
3.4. NO2-OA attenuates hepatic triglyceride accumulation in DIO
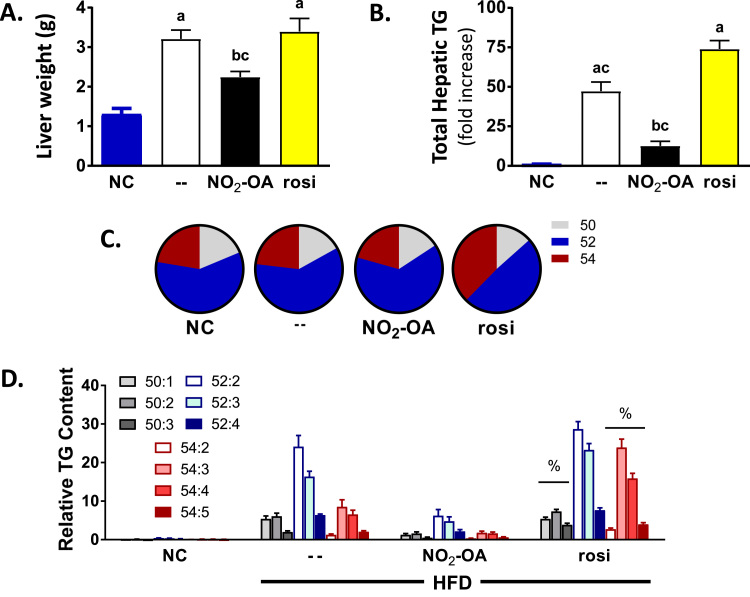
The liver weight in both the vehicle and rosiglitazone treated HFD mice was significantly greater than NC+vehicle and NO2-OA treated HFD mice (b and c refer to p < 0.05 vs HFD-vehicle and -rosiglitazone groups, respectively) in Fig. 3A. The changes in liver weights at the time of sacrifice in the different treatment groups correlated with total hepatic TG content. Despite an improvement in glucose homeostasis and significantly decreased plasma ALT concentrations compared to HFD vehicle group, the total amount of hepatic TGs increased 74-fold in the rosiglitazone group compared to the NC mice. Notably, rosiglitazone-treated mice had a 64% increase in total hepatic TG compared to HFD vehicle treated mice (Fig. 3B). There was no statistically significant difference in total hepatic TGs between vehicle-treated NC mice and HFD-treated mice receiving NO2-OA. Specifically, 6 wk of NO2-OA treatment at the end of 20 wk of HFD significantly decreased total hepatic TG 3.8- and 5.9-fold compared to HFD treated with vehicle or rosi, respectively. Moreover, NO2-OA treatment significantly decreased plasma ALT levels and prevented liver damage induced by HFD (Table 1).
Fig. 3.
NO2-OA treatment significantly decreases total hepatic triglycerides compared to HFD mice treated with vehicle or rosi. (A) The weight of the liver at sacrifice. (20 wk). (B) The total hepatic TG content was normalized to the age-matched NC group and graphed as a fold increase over NC. (C) Specific TG species were identified based on carbon length and saturation. For example, TG 52:2 represents a molecular mass consistent with either a combination of 16:0/18:1/18:1 or 16:0/18:0/18:2. All combinations of TGs were initially screened as ammonium adducts by calculated MRM transitions ranging from C42 to C54 (which contain 10:0, 12:0, 14:0, 14:1, 16:0, 16:1, 18:0, 18:1, 18:2, 18:3 and 20:0 fatty acids). The most predominant hepatic TGs species, C50 to C54, were further characterized to establish the fatty acid composition. (D) The relative abundance of TG species was calculated by the peak area ratio (analyte/internal standard) normalized for milligrams of liver protein. All the data are the mean ± SEM. One-way ANOVA was used and significance was determined as: a, p < 0.05 vs NC; b, p < 0.05 vs HFD mice treated with vehicle; c, p < 0.05 vs rosi-treated HFD mice; %, p < 0.05 vs all groups and treatment.
3.5. Rosiglitazone, but not NO2-OA, changes overall TG profile
Specific TG species were identified based on carbon length and saturation. For example, TG 52:2 consists of either a combination of 16:0/18:1/18:1 or 16:0/18:0/18:2. All combinations of TGs were initially screened as ammonium adducts by calculated MRM transitions ranging from C42 to C54 (which contain 10:0, 12:0, 14:0, 14:1, 16:0, 16:1, 18:0, 18:1, 18:2, 18:3 and 20:0 fatty acids) [41]. The most predominant hepatic TGs species were C50 to C54 and were further characterized to establish the fatty acid composition. The composition of the hepatic TGs was normalized by percent. There was a consistent presence of the relative distribution profile of these TG species that only differed in magnitude in both diet and treatment, except for the rosiglitazone group (Fig. 3C). Specifically, NC with vehicle, HFD+vehicle and HFD+NO2-OA had similar hepatic TG species profiles. Whereas, HFD mice treated with rosiglitazone shifted the composition of fatty acid in the hepatic TGs from shorter chain (and less saturation) to longer chain fatty acids with more double bonds (%, p < 0.05 vs all the groups) in Fig. 3C and D.
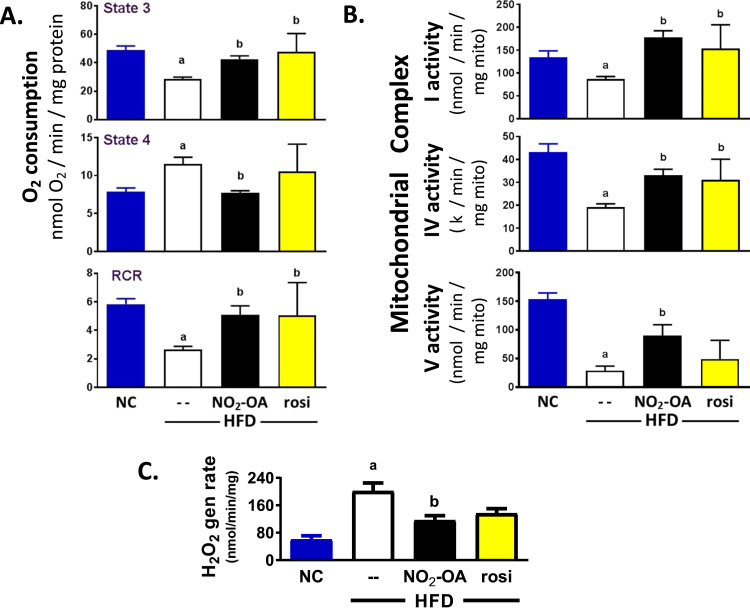
3.6. NO2-OA improves hepatic mitochondrial function in mice fed a HFD
Obesity and insulin resistance are associated with mitochondrial dysfunction [48], [49]. To ascertain whether mitochondrial function is impaired in this model, mitochondrial respiration and the activity of individual respiratory chain complexes was examined. Mitochondrial respiration was affected by DIO. State 3 respiration was significantly decreased in the vehicle HFD group compared to NC mice (28.5 ± 1.4 vs 49.0 ± 2.9 nmol O2/min/mg protein; a, p < 0.05 vs NC, Fig. 4A top). Conversely, state 4 respiration was significantly increased in HFD compared to NC mice (11.5 ± 0.9 vs 7.9 ± 0.5 nmol O2/min/mg protein; a, p < 0.05 vs NC, Fig. 4A middle). This decreased state 3 and increased state 4 respiration translated into a significant decrease in the respiratory control ratio (RCR) of the HFD versus NC mice (2.7 ± 0.2 vs 5.8 ± 0.4; a, p < 0.05 vs NC), Fig. 4A bottom. Both NO2-OA and rosiglitazone treatment protected against DIO-mediated decreases in RCR. Administration of NO2-OA to HFD mice, significantly restored state 3 respiration and completely normalized state 4 respiration (b, p < 0.05 vs HFD+vehicle). Rosiglitazone had a similar effect, with a significant increase in state 3 respiration and no change in state 4 (although high variability) compared to HFD-treated mice.
Fig. 4.
NO2-OA protects against obesity-induced mitochondrial dysfunction. (A) State 3 and 4 respiration was measured in freshly isolated liver mitochondria using glutamate/malate as oxidizable substrates and the RCR was calculated. (B) The rotenone-sensitive rate of NADH oxidation (Complex I) was assessed. Complex IV activity was determined by monitoring the rate of oxidation of fully reduced cytochrome c at 550 nm in the presence/absence of cyanide. Complex V activity assessed the ability to generate ATP (in the presence of ADP). (C) The rate of H2O2 generation was determined via Amplex Red oxidation by isolated hepatic mitochondria. All the data are the mean ± SEM. One-way ANOVA was used and significance was determined as: a, p < 0.05 vs NC; b, p < 0.05 vs HFD mice treated with vehicle.
Net oxygen consumption is a composite measure of the function of each respiratory complex, thus the activity of each complex was measured to identify where the impact of HFD and drug interventions was located. Mice on this HFD displayed a lower hepatic mitochondrial complex I (rotenone sensitive rate of NADH oxidation), IV (cytochrome c oxidase) and V activity compared to mice on NC for 20 wks (Fig. 4B). Treatment with NO2-OA for the last 6 wk of the HFD significantly limited HFD-induced decreases in mitochondrial complex I, IV and V activity. Moreover, rosiglitazone treatment had similar effects in normalizing mitochondrial respiratory complex activities, despite providing no apparent improvement in the steatotic state (Fig. 3). In all treatment groups there was no significant change in mitochondrial protein expression in complex I, IV and V (not shown). Finally, isolated liver mitochondrial hydrogen peroxide (H2O2) generation rates revealed that NO2-OA treated HFD mice had a significant decrease in H2O2 generation rate compared to vehicle (115.9 ± 13.8 vs 201 ± 24.2 nmol/min/mg, Fig. 4C).
4. Discussion
This murine model of diet-induced obesity and fatty liver disease reveals that 6 wk of NO2-OA administration improves glucose tolerance and decreases hepatic TG accumulation. Comparisons with a drug often administered to a related patient population (rosiglitazone) showed a) both NO2-OA and rosiglitazone prevented HFD-induced liver damage (determined by plasma ALT), improved glucose homeostasis and mitochondrial function and b) rosiglitazone increased TG accumulation in the HFD mice. This difference may be due to further rosiglitazone-induced weight gain, one of the adverse effects associated with TZDs [50], [51], [52], [53], [54]. The HFD-induced increases in body weight and fat mass was not observed in the NO2-OA treated mice compared to vehicle.
Rosiglitazone is a potent full agonist of PPARγ that induces adipocyte differentiation. It is generally assumed that this activation is responsible for increased fat mass and body weight gain [55], [56], [57]. We reported that leptin deficient (ob/ob) mice given rosiglitazone had accelerated body weight gain and increased adipogenesis and that these adverse side effects were not observed following NO2-OA treatment [26]. Both rosiglitazone and NO2-OA bind PPARγ with high affinity. The central difference is that NO2-OA acts a partial agonist by covalently adducting to Cys285 in the ligand binding domain of PPARγ. This results in minimal conformational change (in helix 12), thus promoting only partial coactivator recruitment and weak corepressor displacement [26], [58]. In contrast, rosiglitazone induces a full PPARγ agonist response, characterized by a strong co-activator recruitment and full co-repressor displacement, while also inducing potent transactivation and targeted gene expression [26], [58]. This full PPARγ response is strongly linked to adverse effects, despite being potent insulin sensitizers for treating patients with type 2 diabetes [59]. The main goal of diabetes treatment is to not only control blood glucose levels, but to also protect against its comorbidities (such as steatosis), therefore finding a therapeutic agent that has similar insulin sensitizing effects as TZDs without adverse effects remains an unmet need.
We tested the effects of NO2-OA on hepatic steatosis in a more clinically-relevant diet induced obesity model. Typically, obesity increases PPARγ- 1 and - 2 mRNA expression levels in the liver of animal models, an event observed clinically [60], [61], [62], [63], [64], [65] and considered a prosteatotic factor in NAFLD [66]. In non-obese mice, hepatic overexpression of PPARγ significantly increases TG accumulation that co-localizes only with hepatocytes expressing PPARγ [63]. When PPARγ expression is high in the liver, rosiglitazone treatment increases neutral lipid accumulation [63]. In the current study, rosiglitazone treatment of HFD mice increased hepatic PPARγ mRNA expression 3.5-fold over NC (p = 0.0339); whereas there was no increase in PPARγ expression with NO2-OA (0.73-fold vs NC, p = 0.9477, not shown). This data suggests that the increase in hepatic PPARγ expression with rosiglitazone treatment may play a role in the increased TG accumulation.
Obesity and insulin resistance is characterized by chronic nutritional overload [67] and ultimately leads to ectopic fat accumulation and increased de novo lipogenesis rates in the liver [68], [69]. Lipotoxicity and increased substrate overload leads to mitochondrial adaptations and remodeling in obesity, insulin resistance and steatosis in both patients and preclinical models [70], [71], [72]. Initially, mitochondria compensate for excess substrate/lipid load by increasing mitochondrial oxidative bioenergetics. Eventually, compensatory adaptations cannot overcome substrate overload and lipotoxicity, resulting in inefficient and incomplete β-oxidation and the accumulation of hepatic ceramides, diacylglycerols (DAGs) and long-chain acylcarnitines [73]. There is a direct causal role of increased ceramides, DAGs and long-chain acylcarnitines, due to suboptimal fat oxidation in hepatic insulin resistance and steatosis. This occurs by stimulating inflammatory pathways, generating reactive species and interfering with hepatic insulin signaling [74], [75]. We previously reported that NO2-OA improves adipose tissue function, an effect characterized by a significant decrease in inflammatory signaling mediators and reactive species generation (xanthine oxidase activity), as well as normalization of circulating adipokines (leptin and adiponectin) [38]. Indicative of improved adipose tissue function, the circulating NEFA in the NO2-OA treatment group were decreased compared to vehicle-treated HFD mice (0.44 ± 0.028 vs 0.54 ± 0.034; p = 0.0288, unpaired t-test). Weight loss also improves the steatotic state in patients with NASH and in preclinical animal models. The improved adipose tissue function decreased circulating NEFA concentrations and this, in turn, alleviates the overall FFA load on the liver. As there was no difference in weight between HFD mice treated with vehicle or NO2-OA, the improvement in glucose tolerance and hepatic TG accumulation observed after NO2-OA administration is likely due to a number of events being instigated by this pleiotropic mediator that requires further investigation.
In this study, DIO resulted in impaired mitochondrial function, characterized by a decrease in state 3 respiration (max ATP/coupled respiration) with glutamate/malate used as substrates, and an increase in state 4 respiration (proton leak). This translates into a significant decrease in the RCR (2.66 ± 0.2), a measure of respiratory efficiency. Additionally, HFD feeding significantly decreased mitochondrial complex I, IV and V activity (Fig. 4B). There was no difference in citrate synthase activity, commonly considered a mitochondrial housekeeping enzyme and a surrogate for mitochondrial number between all the treatment groups (not shown). These observations indicate that mitochondrial respiration is significantly impaired and not efficient in generating ATP via oxidative phosphorylation. This data set is consistent with other studies where diet- and genetically-induced obesity causes mitochondrial dysfunction [60], [76]. Whether this is true mitochondrial dysfunction or simply an adaptation to the increased nutritional overload remains to be investigated. Notably, impaired mitochondrial function also occurs in patients with NASH [77]. Characterizing and understanding the basis for this bioenergetic pathophysiology will help address why some are predisposed to NASH and will also reveal new pathways to target with novel therapeutics.
The direct impact of NO2-OA on hepatic mitochondrial function in vivo remains undefined and is difficult to determine in models of obesity. Previous studies have shown that NO2-FAs are protective against ischemia/reperfusion injury by inducing mild mitochondrial uncoupling via post-translational modifications (PTMs) of adenine nucleotide translocase (ANT) and uncoupling protein (UCP)-2 [78]. UCPs and ANT dissipate the ETC proton gradient, allowing energy to be released as heat, a process that concomitantly decreases superoxide (O2•-) production and suppresses oxidative damage [79]. Additionally, NO2-OA inhibits complex II-linked respiration in isolated rat heart mitochondria and suppresses O2•- formation [80]. Taken together, NO2-FAs confer cardioprotection in ischemia/reperfusion injury models by altering mitochondrial function. It is unknown if NO2-OA induces mild uncoupling in our DIO model, though the decrease in RCR observed here is consistent with uncoupling. More importantly, NO2-OA attenuated HFD-induced mitochondrial H2O2 generation (Fig. 4C). Collectively, these findings demonstrate that NO2-FAs decrease reactive species generation and herein, may provide mitochondrial protection against HFD-induced substrate overload.
Rosiglitazone treatment showed a decrease in circulating NEFA and ALT compared to the HFD-vehicle group. Yet, there was still a robust increase in hepatic TG accumulation. The present data reaffirms that rosiglitazone exacerbates hepatic steatosis [60], [61], [62], [63] even with improved glucose tolerance (Fig. 2) and no liver damage as reflected by plasma ALT (Table 1). More in depth analysis revealed that the overall hepatic TG profile was similar between NC, HFD+vehicle and HFD+NO2-OA but not with the HFD+rosiglitazone group. The hepatic TGs of the rosiglitazone treated HFD mice had a higher content of more unsaturated longer chain fatty acids (Fig. 3C). The esterification of more longer chain unsaturated fatty acids may have implications in downstream signaling and provide additional evidence as to why rosiglitazone is a potent insulin sensitizer even in the presence of hepatic steatosis. Despite the significant increase in hepatic TG accumulation, rosiglitazone only modestly limited HFD-induced mitochondrial dysfunction. This normalization of mitochondrial function was not observed in ob/ob mice, where rosiglitazone decreased complex I activity and failed to alter activity of any of the other complexes (II-V) compared to vehicle [60]. Notably, rosiglitazone treatment increases weight gain and changes the distribution of lipids in hepatic TGs. This was not observed in the NO2-OA-treated mice and may contribute to the observed improvement in mitochondrial function.
Our results demonstrate that rosiglitazone improves glucose tolerance but did not protect against hepatic steatosis, which begs the question of the benefits of improving insulin sensitivity in the presence of hepatic steatosis. This motivates consideration as to whether it is more advantageous to improve insulin sensitivity or hepatic steatosis. This is why it is imperative to find a novel therapeutic for the treatment of hepatic steatosis without detrimental side effects, in particular weight gain.
In summary, this is the first demonstration that NO2-OA decreases hepatic TG accumulation in a HFD model. Both rosiglitazone and NO2-OA prevented liver damage, improved glucose tolerance and mitochondrial function. Rosiglitazone promoted increased fat mass, weight gain and hepatic TG accumulation. This preclinical data suggest that NO2-OA or other electrophilic species could be a viable therapeutic agent for obesity without adverse side effects.
Acknowledgements
This study was supported by National Institutes of Health Grants R01-HL64937, R01-HL132550 and P01-HL103455 (B.A.F.) and University of Pittsburgh Medical Center Competitive Medical Research Fund Award (N.K.H.K.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
BAF has financial interest in Complexa Inc. All other authors declare that they have no conflicts of interest with the contents of this article.
Contributor Information
Nicholas K.H. Khoo, Email: nkhoo@pitt.edu.
Bruce A. Freeman, Email: freerad@pitt.edu.
References
- 1.Li L., Liu D.W., Yan H.Y., Wang Z.Y., Zhao S.H., Wang B. Obesity is an independent risk factor for non‐alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes. Rev. 2016;17:510–519. doi: 10.1111/obr.12407. [DOI] [PubMed] [Google Scholar]
- 2.Bellentani S., Scaglioni F., Marino M., Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig. Dis. 2010;28:155–161. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 3.Vernon G., Baranova A., Younossi Z.M. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 4.Promrat K., Kleiner D.E., Niemeier H.M., Jackvony E., Kearns M., Wands J.R., Fava J., Wing R.R. Randomized controlled trial testing the Effects of weight loss on nonalcoholic steatohepatitis (NASH) Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naga C., Zobair Y., LJ E., Mae D.A., BE M., Kenneth C., Michael C., SA J. The diagnosis and management of non‐alcoholic fatty liver disease: practice Guideline by the American Association for the study of liver diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 6.HS A., Will F., BE M., NTB A. Orlistat for overweight subjects with nonalcoholic steatohepatitis: a randomized, prospective trial. Hepatology. 2009;49:80–86. doi: 10.1002/hep.22575. [DOI] [PubMed] [Google Scholar]
- 7.Vilar-Gomez E., Martinez-Perez Y., Calzadilla-Bertot L., Torres-Gonzalez A., Gra-Oramas B., Gonzalez-Fabian L., Friedman S.L., Diago M., Romero-Gomez M. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(367–78):e5. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Ratziu V. Pharmacological agents for NASH. Nat. Rev. Gastroenterol. ; Hepatol. 2013;10:676. doi: 10.1038/nrgastro.2013.193. [DOI] [PubMed] [Google Scholar]
- 9.Utzschneider K.M., Kahn S.E. The role of insulin resistance in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2006;91:4753–4761. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 10.Byrne C.D., Targher G. NAFLD: a multisystem disease. J. Hepatol. 2015;62:S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Gruben N., Shiri-Sverdlov R., Koonen D.P.Y., Hofker M.H. Nonalcoholic fatty liver disease: a main driver of insulin resistance or a dangerous liaison? Biochim. Et. Biophys. Acta (BBA) - Mol. Basis Dis. 2014;1842:2329–2343. doi: 10.1016/j.bbadis.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Mayerson A.B., Hundal R.S., Dufour S., Lebon V., Befroy D., Cline G.W., Enocksson S., Inzucchi S.E., Shulman G.I., Petersen K.F. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51:797. doi: 10.2337/diabetes.51.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyazaki Y., Mahankali A., Matsuda M., Mahankali S., Hardies J., Cusi K., Mandarino L.J., DeFronzo R.A. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 2002;87:2784–2791. doi: 10.1210/jcem.87.6.8567. [DOI] [PubMed] [Google Scholar]
- 14.Nesto R.W., Bell D., Bonow R.O., Fonseca V., Grundy S.M., Horton E.S., Le W.M., Porte D., Semenkovich C.F., Smith S., Young L.H., Kahn R. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation. 2003;108:2941–2948. doi: 10.1161/01.CIR.0000103683.99399.7E. [DOI] [PubMed] [Google Scholar]
- 15.Lincoff A.M., Wolski K., Nicholls S.J., Nissen S.E. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus. JAMA: J. Am. Med. Assoc. 2007;298:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 16.Nissen S.E., Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 17.Shadid S., Jensen M.D. Effects of pioglitazone versus diet and exercise on metabolic health and fat distribution in upper body obesity. Diabetes Care. 2003;26:3148. doi: 10.2337/diacare.26.11.3148. [DOI] [PubMed] [Google Scholar]
- 18.Reddy A.T., Lakshmi S.P., Dornadula S., Pinni S., Rampa D.R., Reddy R.C. The nitrated fatty acid 10-nitro-oleate attenuates allergic airway disease. J. Immunol. 2013;191:2053–2063. doi: 10.4049/jimmunol.1300730. [DOI] [PubMed] [Google Scholar]
- 19.Rudolph T.K., Rudolph V., Edreira M.M., Cole M.P., Bonacci G., Schopfer F.J., Woodcock S.R., Franek A., Pekarova M., Khoo N.K., Hasty A.H., Baldus S., Freeman B.A. Nitro-fatty acids reduce atherosclerosis in apolipoprotein e-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2010;30:938–945. doi: 10.1161/ATVBAHA.109.201582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y., Jia Z., Liu S., Downton M., Liu G., Du Y., Yang T. Combined losartan and nitro-oleic acid remarkably improves diabetic nephropathy in mice. Am. J. Physiol. Ren. Physiol. 2013;305:F1555–F1562. doi: 10.1152/ajprenal.00157.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H., Jia Z., Soodvilai S., Guan G., Wang M.H., Dong Z., Symons J.D., Yang T. Nitro-oleic acid protects the mouse kidney from ischemia and reperfusion injury. Am. J. Physiol. Ren. Physiol. 2008;295:F942–F949. doi: 10.1152/ajprenal.90236.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villacorta L., Chang L., Salvatore S.R., Ichikawa T., Zhang J., Petrovic-Djergovic D., Jia L., Carlsen H., Schopfer F.J., Freeman B.A., Chen Y.E. Electrophilic nitro-fatty acids inhibit vascular inflammation by disrupting LPS-dependent TLR4 signalling in lipid rafts. Cardiovasc. Res. 2013;98:116–124. doi: 10.1093/cvr/cvt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H., Liu H., Jia Z., Olsen C., Litwin S., Guan G., Yang T. Nitro-oleic acid protects against endotoxin-induced endotoxemia and multiorgan injury in mice. Am. J. Physiol. Ren. Physiol. 2010;298:F754–F762. doi: 10.1152/ajprenal.00439.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J., Villacorta L., Chang L., Fan Z., Hamblin M., Zhu T., Chen C.S., Cole M.P., Schopfer F.J., Deng C.X., Garcia-Barrio M.T., Feng Y.H., Freeman B.A., Chen Y.E. Nitro-oleic acid inhibits angiotensin II-induced hypertension. Circ. Res. 2010;107:540–548. doi: 10.1161/CIRCRESAHA.110.218404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charles R.L., Rudyk O., Prysyazhna O., Kamynina A., Yang J., Morisseau C., Hammock B.D., Freeman B.A., Eaton P. Protection from hypertension in mice by the Mediterranean diet is mediated by nitro fatty acid inhibition of soluble epoxide hydrolase. Proc. Natl. Acad. Sci. USA. 2014;111:8167–8172. doi: 10.1073/pnas.1402965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schopfer F.J., Cole M.P., Groeger A.L., Chen C.S., Khoo N.K., Woodcock S.R., Golin-Bisello F., Motanya U.N., Li Y., Zhang J., Garcia-Barrio M.T., Rudolph T.K., Rudolph V., Bonacci G., Baker P.R., Xu H.E., Batthyany C.I., Chen Y.E., Hallis T.M., Freeman B.A. Covalent peroxisome proliferator-activated receptor γ binding by nitro-fatty acids: endogenous ligands act as selective modulators. J. Biol. Chem. 2010;285:12321–12333. doi: 10.1074/jbc.M109.091512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klinke A., Moller A., Pekarova M., Ravekes T., Friedrichs K., Berlin M., Scheu K.M., Kubala L., Kolarova H., Ambrozova G., Schermuly R.T., Woodcock S.R., Freeman B.A., Rosenkranz S., Baldus S., Rudolph V., Rudolph T.K. Protective effects of 10-nitro-oleic acid in a hypoxia-induced murine model of pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 2014;51:155–162. doi: 10.1165/rcmb.2013-0063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelley E.E., Baust J., Bonacci G., Golin-Bisello F., Devlin J.E., St Croix C.M., Watkins S.C., Gor S., Cantu-Medellin N., Weidert E.R., Frisbee J.C., Gladwin M.T., Champion H.C., Freeman B.A., Khoo N.K. Fatty acid nitroalkenes ameliorate glucose intolerance and pulmonary hypertension in high-fat diet-induced obesity. Cardiovasc. Res. 2014;101:352–363. doi: 10.1093/cvr/cvt341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.〈http://clinicaltrials.gov/ct2/results?Term=cxa-10&Search=Search〉. US National Institutes of Health.
- 30.Cui T., Schopfer F.J., Zhang J., Chen K., Ichikawa T., Baker P.R., Batthyany C., Chacko B.K., Feng X., Patel R.P., Agarwal A., Freeman B.A., Chen Y.E. Nitrated fatty acids: endogenous anti-inflammatory signaling mediators. J. Biol. Chem. 2006;281:35686–35698. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodcock C.-S.C., Huang Y., Woodcock S.R., Salvatore S.R., Singh B., Golin-Bisello F., Davidson N.E., Neumann C.A., Freeman B.A., Wendell S.G. Nitro-fatty acid inhibition of triple-negative breast cancer cell viability, migration, invasion, and tumor growth. J. Biol. Chem. 2018;293:1120–1137. doi: 10.1074/jbc.M117.814368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kansanen E., Bonacci G., Schopfer F.J., Kuosmanen S.M., Tong K.I., Leinonen H., Woodcock S.R., Yamamoto M., Carlberg C., Ylä-Herttuala S., Freeman B.A., Levonen A.-L. Electrophilic nitro-fatty acids activate NRF2 by a KEAP1 cysteine 151-independent mechanism. J. Biol. Chem. 2011;286:14019–14027. doi: 10.1074/jbc.M110.190710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudolph T.K., Ravekes T., Klinke A., Friedrichs K., Mollenhauer M., Pekarova M., Ambrozova G., Martiskova H., Kaur J.-J., Matthes B., Schwoerer A., Woodcock S.R., Kubala L., Freeman B.A., Baldus S., Rudolph V. Nitrated fatty acids suppress angiotensin II-mediated fibrotic remodelling and atrial fibrillation. Cardiovasc. Res. 2016;109:174–184. doi: 10.1093/cvr/cvv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddy A.T., Lakshmi S.P., Zhang Y., Reddy R.C. Nitrated fatty acids reverse pulmonary fibrosis by dedifferentiating myofibroblasts and promoting collagen uptake by alveolar macrophages. FASEB J. 2014;28:5299–5310. doi: 10.1096/fj.14-256263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charles R.L., Rudyk O., Prysyazhna O., Kamynina A., Yang J., Morisseau C., Hammock B.D., Freeman B.A., Eaton P. Protection from hypertension in mice by the Mediterranean diet is mediated by nitro fatty acid inhibition of soluble epoxide hydrolase. Proc. Natl. Acad. Sci. USA. 2014 doi: 10.1073/pnas.1402965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.González-Perilli L., Álvarez M.N., Prolo C., Radi R., Rubbo H., Trostchansky A. Nitroarachidonic acid prevents NADPH oxidase assembly and superoxide radical production in activated macrophages. Free Radic. Biol. Med. 2013;58:126–133. doi: 10.1016/j.freeradbiomed.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelley E.E., Batthyany C.I., Hundley N.J., Woodcock S.R., Bonacci G., Del Rio J.M., Schopfer F.J., Lancaster J.R., Freeman B.A., Tarpey M.M. Nitro-oleic acid, a novel and irreversible inhibitor of Xanthine Oxidoreductase. J. Biol. Chem. 2008;283:36176–36184. doi: 10.1074/jbc.M802402200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelley E.E., Baust J., Bonacci G., Golin-Bisello F., Devlin J.E., St. Croix C.M., Watkins S.C., Gor S., Cantu-Medellin N., Weidert E.R., Frisbee J.C., Gladwin M.T., Champion H.C., Freeman B.A., Khoo N.K.H. Fatty acid nitroalkenes ameliorate glucose intolerance and pulmonary hypertension in high fat diet-induced obesity. Cardiovasc. Res. 2014;101:352–363. doi: 10.1093/cvr/cvt341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergheim I., Guo L., Davis M.A., Lambert J.C., Beier J.I., Duveau I., Luyendyk J.P., Roth R.A., Arteel G.E. Metformin prevents alcohol-induced liver injury in the mouse: critical role of plasminogen activator inhibitor-1. Gastroenterology. 2006;130:2099–2112. doi: 10.1053/j.gastro.2006.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 41.Chartoumpekis D.V., Palliyaguru D.L., Wakabayashi N., Fazzari M., Khoo N.K.H., Schopfer F.J., Sipula I., Yagishita Y., Michalopoulos G.K., O’Doherty R.M., Kensler T.W. Nrf2 deletion from adipocytes, but not hepatocytes, potentiates systemic metabolic dysfunction after long-term high-fat diet-induced obesity in mice. Am. J. Physiol.-Endocrinol. Metab. 2018;315:E180–E195. doi: 10.1152/ajpendo.00311.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chartoumpekis D.V., Yagishita Y., Fazzari M., Palliyaguru D.L., Rao U.N.M., Zaravinos A., Khoo N.K.H., Schopfer F.J., Weiss K.R., Michalopoulos G.K., Sipula I., O’Doherty R.M., Kensler T.W., Wakabayashi N. Nrf2 prevents Notch-induced insulin resistance and tumorigenesis in mice. JCI Insight. 2018;3:e97735. doi: 10.1172/jci.insight.97735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiva S., Rassaf T., Patel R.P., Gladwin M.T. The detection of the nitrite reductase and NO-generating properties of haemoglobin by mitochondrial inhibition. Cardiovasc. Res. 2011;89:566–573. doi: 10.1093/cvr/cvq327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiva S., Sack M.N., Greer J.J., Duranski M., Ringwood L.A., Burwell L., Wang X., MacArthur P.H., Shoja A., Raghavachari N., Calvert J.W., Brookes P.S., Lefer D.J., Gladwin M.T. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J. Exp. Med. 2007;204:2089. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cardenes N., Corey C., Geary L., Jain S., Zharikov S., Barge S., Novelli E.M., Shiva S. Platelet bioenergetic screen in sickle cell patients reveals mitochondrial complex V inhibition, which contributes to platelet activation. Blood. 2014;123:2864–2872. doi: 10.1182/blood-2013-09-529420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wharton D.C., Tzagoloff A. Methods in Enzymology. Academic Press; 1967. Cytochrome oxidase from beef heart mitochondria mitochondria; pp. 245–250. [Google Scholar]
- 47.Mo L., Wang Y., Geary L., Corey C., Alef M.J., Beer-Stolz D., Zuckerbraun B.S., Shiva S. Nitrite activates AMP kinase to stimulate mitochondrial biogenesis independent of soluble guanylate cyclase. Free Radic. Biol. Med. 2012;53:1440–1450. doi: 10.1016/j.freeradbiomed.2012.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lowell B.B., Shulman G.I. Mitochondrial Dysfunction and Type 2 Diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 49.Mantena S.K., King A.L., Andringa K.K., Eccleston H.B., Bailey S.M. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic. Biol. Med. 2008;44:1259–1272. doi: 10.1016/j.freeradbiomed.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raskin P., Rendell M., Riddle M.C., Dole J.F., Freed M.I., Rosenstock J. A randomized trial of rosiglitazone therapy in patients with inadequately controlled insulin-treated type 2 diabetes. Diabetes Care. 2001;24:1226. doi: 10.2337/diacare.24.7.1226. [DOI] [PubMed] [Google Scholar]
- 51.Balas B., Belfort R., Harrison S.A., Darland C., Finch J., Schenker S., Gastaldelli A., Cusi K. Pioglitazone treatment increases whole body fat but not total body water in patients with non-alcoholic steatohepatitis. J. Hepatol. 2007;47:565–570. doi: 10.1016/j.jhep.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 52.Takazawa T., Yamauchi T., Tsuchida A., Takata M., Hada Y., Iwabu M., Okada-Iwabu M., Ueki K., Kadowaki T. Peroxisome proliferator-activated receptor γ agonist rosiglitazone increases expression of very low density lipoprotein receptor gene in adipocytes. J. Biol. Chem. 2009;284:30049–30057. doi: 10.1074/jbc.M109.047993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Souza C.J., Eckhardt M., Gagen K., Dong M., Chen W., Laurent D., Burkey B.F. Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. Diabetes. 2001;50:1863. doi: 10.2337/diabetes.50.8.1863. [DOI] [PubMed] [Google Scholar]
- 54.Chao L., Marcus-Samuels B., Mason M.M., Moitra J., Vinson C., Arioglu E., Gavrilova O., Reitman M.L. Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones. J. Clin. Investig. 2000;106:1221–1228. doi: 10.1172/JCI11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haliakon S., Doaré L., Foufelle F., Kergoat M., Guerre-Millo M., Berthault M.-F., Dugail I., Morin J., Auwerx J., Ferré P. Pioglitazone induces In vivo adipocyte differentiation in the obese Zucker fa/fa rat. Diabetes. 1997;46:1393. doi: 10.2337/diab.46.9.1393. [DOI] [PubMed] [Google Scholar]
- 56.Kubota N., Terauchi Y., Yamauchi T., Kubota T., Moroi M., Matsui J., Eto K., Yamashita T., Kamon J., Satoh H., Yano W., Froguel P., Nagai R., Kimura S., Kadowaki T., Noda T. Disruption of adiponectin causes insulin resistance and neointimal formation. J. Biol. Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 57.Moore G.B.T., Chapman H., Holder J.C., Lister C.A., Piercy V., Smith S.A., Clapham J.C. Differential regulation of adipocytokine mRNAs by rosiglitazone in db/db Mice. Biochem. Biophys. Res. Commun. 2001;286:735–741. doi: 10.1006/bbrc.2001.5460. [DOI] [PubMed] [Google Scholar]
- 58.Li Y., Zhang J., Schopfer F.J., Martynowski D., Garcia-Barrio M.T., Kovach A., Suino-Powell K., Baker P.R., Freeman B.A., Chen Y.E., Xu H.E. Molecular recognition of nitrated fatty acids by PPAR gamma. Nat. Struct. Mol. Biol. 2008;15:865–867. doi: 10.1038/nsmb.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia-Vallvé S., Guasch L., Tomas-Hernández S., del Bas J.M., Ollendorff V., Arola L., Pujadas G., Mulero M. Peroxisome proliferator-activated receptor γ (PPARγ) and ligand choreography: newcomers take the stage. J. Med. Chem. 2015;58:5381–5394. doi: 10.1021/jm501155f. [DOI] [PubMed] [Google Scholar]
- 60.García-Ruiz I., Rodríguez-Juan C., Díaz-Sanjuán T., Martínez M.Á., Muñoz-Yagüe T., Solís-Herruzo J.A. Effects of rosiglitazone on the liver histology and mitochondrial function in ob/ob mice. Hepatology. 2007;46:414–423. doi: 10.1002/hep.21687. [DOI] [PubMed] [Google Scholar]
- 61.Rull A., Geeraert B., Aragonès G., Beltrán-Debón R., Rodríguez-Gallego E., García-Heredia A., Pedro-Botet J., Joven J., Holvoet P., Camps J. Rosiglitazone and fenofibrate exacerbate liver steatosis in a mouse model of obesity and hyperlipidemia. A transcriptomic and metabolomic study. J. Proteome Res. 2014;13:1731–1743. doi: 10.1021/pr401230s. [DOI] [PubMed] [Google Scholar]
- 62.Bedoucha M., Atzpodien E., Boelsterli U.A. Diabetic KKAy mice exhibit increased hepatic PPARγ1 gene expression and develop hepatic steatosis upon chronic treatment with antidiabetic thiazolidinediones. J. Hepatol. 2001;35:17–23. doi: 10.1016/s0168-8278(01)00066-6. [DOI] [PubMed] [Google Scholar]
- 63.Gao M., Ma Y., Alsaggar M., Liu D. Dual outcomes of rosiglitazone treatment on fatty liver. AAPS J. 2016;18:1023–1031. doi: 10.1208/s12248-016-9919-9. [DOI] [PubMed] [Google Scholar]
- 64.Pettinelli P., Videla L.A. Up-regulation of PPAR-γ mrna expression in the liver of obese patients: an additional reinforcing lipogenic mechanism to SREBP-1c Induction. J. Clin. Endocrinol. Metab. 2011;96:1424–1430. doi: 10.1210/jc.2010-2129. [DOI] [PubMed] [Google Scholar]
- 65.Memon R.A., Tecott L.H., Nonogaki K., Beigneux A., Moser A.H., Grunfeld C., Feingold K.R. Up-regulation of peroxisome proliferator-activated receptors (PPAR-α) and PPAR-γ messenger Ribonucleic acid expression in the liver in murine obesity: Troglitazone induces expression of PPAR-γ-responsive adipose tissue-specific genes in the liver of obese diabetic mice. Endocrinology. 2000;141:4021–4031. doi: 10.1210/endo.141.11.7771. [DOI] [PubMed] [Google Scholar]
- 66.Morán-Salvador E., López-Parra M., García-Alonso V., Titos E., Martínez-Clemente M., González-Périz A., López-Vicario C., Barak Y., Arroyo V., Clària J. Role for PPARγ in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB J. 2011;25:2538–2550. doi: 10.1096/fj.10-173716. [DOI] [PubMed] [Google Scholar]
- 67.Um S.H., D'Alessio D., Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 68.Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fabbrini E., Sullivan S., Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic and clinical implications. Hepatology. 2010;51:679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koliaki C., Szendroedi J., Kaul K., Jelenik T., Nowotny P., Jankowiak F., Herder C., Carstensen M., Krausch M., Knoefel Wolfram T., Schlensak M., Roden M. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21:739–746. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 71.Lambert J.E., Ramos–Roman M.A., Browning J.D., Parks E.J. Increased De Novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:726–735. doi: 10.1053/j.gastro.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koves T.R., Ussher J.R., Noland R.C., Slentz D., Mosedale M., Ilkayeva O., Bain J., Stevens R., Dyck J.R.B., Newgard C.B., Lopaschuk G.D., Muoio D.M. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 73.Patterson R.E., Kalavalapalli S., Williams C.M., Nautiyal M., Mathew J.T., Martinez J., Reinhard M.K., McDougall D.J., Rocca J.R., Yost R.A., Cusi K., Garrett T.J., Sunny N.E. Lipotoxicity in steatohepatitis occurs despite an increase in tricarboxylic acid cycle activity. Am. J. Physiol.-Endocrinol. Metab. 2016;310:E484–E494. doi: 10.1152/ajpendo.00492.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petersen M.C., Shulman G.I. Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends Pharmacol. Sci. 2017;38:649–665. doi: 10.1016/j.tips.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Herpen N.A., Schrauwen-Hinderling V.B. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol. Behav. 2008;94:231–241. doi: 10.1016/j.physbeh.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 76.Mantena S.K., Vaughn D.P., Andringa K.K., Eccleston H.B., King A.L., Abrams G.A., Doeller J.E., Kraus D.W., Darley-Usmar V.M., Bailey S.M. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem. J. 2009;417:183–193. doi: 10.1042/BJ20080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pérez-Carreras M., Del Hoyo P., Martin M.A., Rubio J.C., Martin A., Castellano G., Colina F., Arenas J., Solis-Herruzo J.A. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 2003;38:999–1007. doi: 10.1053/jhep.2003.50398. [DOI] [PubMed] [Google Scholar]
- 78.Nadtochiy S.M., Baker P.R., Freeman B.A., Brookes P.S. Mitochondrial nitroalkene formation and mild uncoupling in ischaemic preconditioning: implications for cardioprotection. Cardiovasc. Res. 2009;82:333–340. doi: 10.1093/cvr/cvn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Azzu V., Brand M.D. The on-off switches of the mitochondrial uncoupling proteins. Trends Biochem. Sci. 2010;35:298–307. doi: 10.1016/j.tibs.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koenitzer J.R., Bonacci G., Woodcock S.R., Chen C.-S., Cantu-Medellin N., Kelley E.E., Schopfer F.J. Fatty acid nitroalkenes induce resistance to ischemic cardiac injury by modulating mitochondrial respiration at complex II. Redox Biol. 2016;8:1–10. doi: 10.1016/j.redox.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]