Fig 1. The presence of 8pCPT alters the number and the diameter of CRVs.
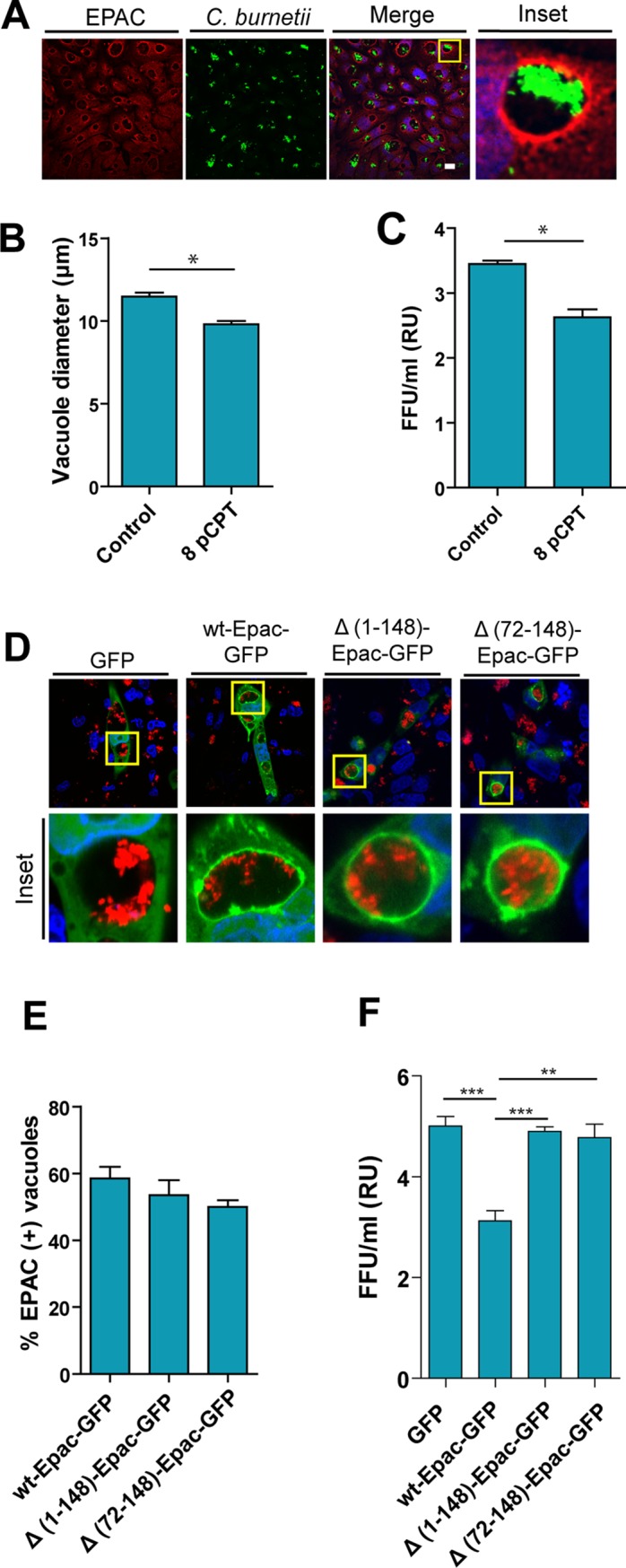
A. CHO cells were infected with C. burnetii for 48 h (MOI = 10), fixed and subjected to indirect immunofluorescence using antibodies to detect endogenous EPAC protein (red) and against C. burnetii to detect the bacteria (green). The cells were analyzed by confocal microscopy. B. Quantification of the diameter of the vacuole in complete medium in the absence (Control) or the presence of 8-pCPT-cAMP (8pCPT). Data represent the mean± SEM of at least three independent experiments in which no less than 200 vacuoles were scored in each experiment. C. CHO cells were incubated in the complete medium in the absence (Control) or presence of 8-pCPT-cAMP (8pCPT) and infected for 48 h with C. burnetii to allow the development of the Coxiella vacuole. The cells were then lysed by sonication and the supernatant was diluted (1:100) and used to infect Vero cells. After 72 h of incubation (chase), cells were fixed and examined by fluorescence microscopy. Data represent the mean ± SEM of at least three independent experiments where a minimum of 1,000 cells were scored in each experiment (*p ≤0.05.). D. CHO cells were transfected with wt-EPAC-GFP, Δ(1–148)-EPAC-GFP, Δ(72–148)-EPAC-GFP and infected with C. burnetii. At 48 h, the cells were fixed and analyzed by confocal microscopy. E. Quantification of the percentage of wt-EPAC-GFP, Δ(1–148)-EPAC-GFP, Δ(72–148)-EPAC-GFP positive vacuoles. F.CHO cells were infected for 48 h with C. burnetii to allow the development of the Coxiella vacuole. The cells were then lysed by sonication and the supernatant was diluted (1:100) and used to infect Vero cells. After 72 h of incubation (chase), cells were fixed and examined by fluorescence microscopy. The data represent the mean ± SEM of at least 3 independent experiments. Tukey test * p ≤ 0.05. Bars = 10 μm.
