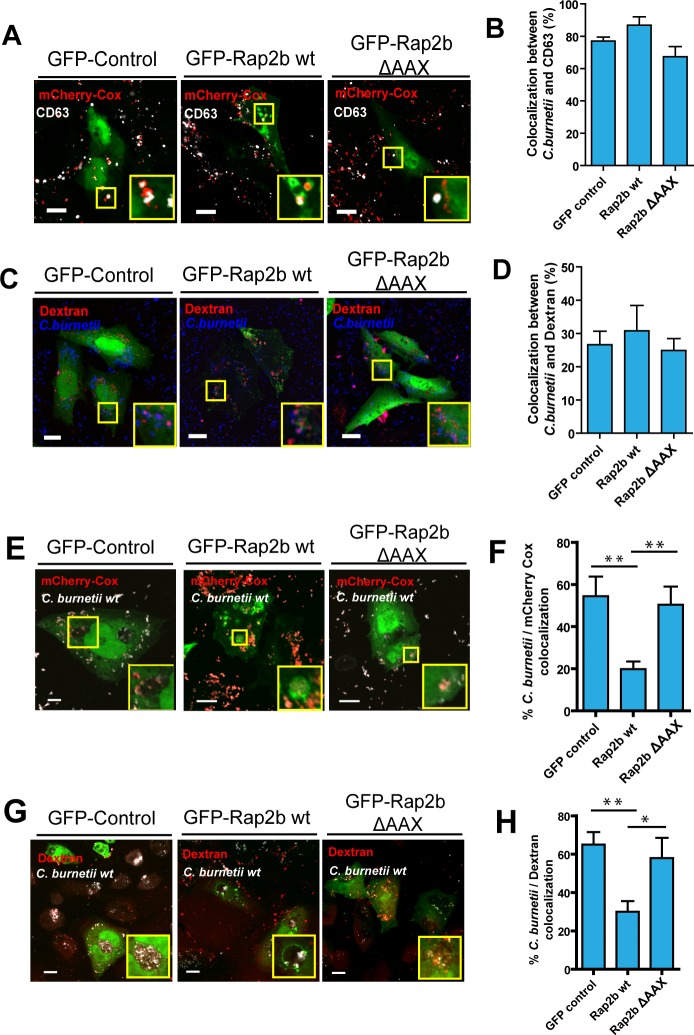
Fig 5. Homotypic fusion between phagosomes containing C. burnetii and the replicative vacuole is altered by overexpression of Rap2b wt at later times of infection.
A. CHO cells were transfected with pEGFP, wt pEGFP-Rap2b or pEGFP-Rap2b ΔAAX. After 24 hours the cells were infected with mCherry-C.burnetii for 2 hours, fixed and subjected to indirect immunofluorescence using antibodies against CD63 (MOI = 10). B. Quantification of colocalization between C burnetii and CD63. C. CHO cells were transfected with pEGFP (control), wt pEGFP-Rap2b or pEGFP-Rap2b ΔAAX and were incubated for 2 hours with 5 μg/ml dextran-rhodamine to allow the labeling of lysosomes. After 24 hours, the cells were infected with C. burnetii for 2 h, fixed and subjected to indirect immunofluorescence using antibodies against C. burnetii (blue). D. Quantification of colocalization between dextran and C. burnetii. The data represent the mean ± SEM of at least 3 independent experiments. Tukey test * p ≤ 0.05. Bars = 10 μm. E. CHO cells were infected with C. burnetii for 24 hours and then transfected with pEGFP, pEGFP-Rap2b or pEGFP-Rap2b ΔAAX. After 24 hours, cells were infected with C. burneti mCherry for 4 h (early phagosomes), fixed and analyzed by confocal microscopy. F. Quantification of the arrival of mCherry-C. burnetii (red) to the vacuole of C. burnetii already formed. G. CHO cells were infected with C. burnetii for 24 h and then transfected with pEGFP, wt pEGFP-Rap2b or pEGFP-Rap2b ΔAAX. After 24 hours, cells were incubated with Texas red dextran for 4 h, fixed and subjected to indirect immunofluorescence using antibodies against C. burnetii (white). H. Quantification of the arrival of Texas red dextran (red) to the vacuole of C. burnetii already formed. The data represent the mean ± SEM of at least 3 independent experiments. Tukey test **p≤0.01, * p ≤0.05. Bars = 10 μm.