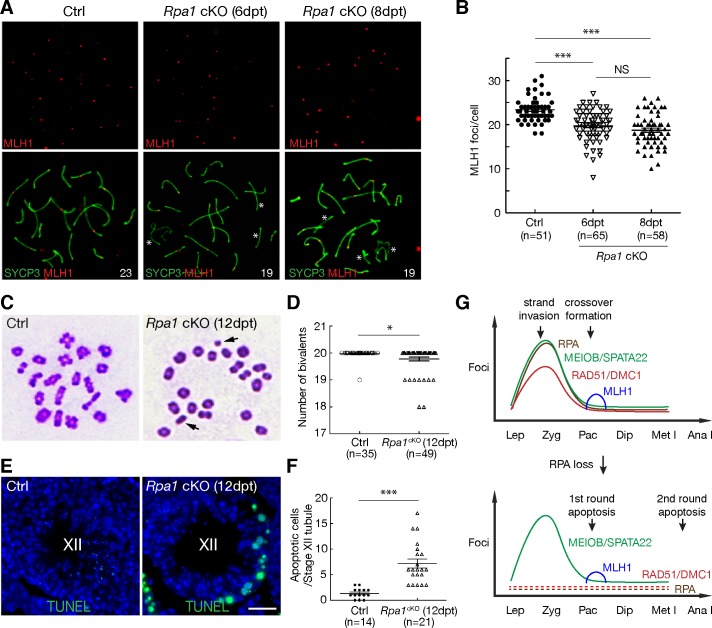
Fig 7. RPA1 regulates the formation of meiotic crossovers.
(A) Reduction in MLH1 foci in Rpa1cKO pachytene spermatocytes. Asterisks mark the chromosomes without MLH1 foci. (B) Counts of MLH1 foci in pachytene spermatocytes. n, number of cells counted from four experiments. (C) Presence of univalent chromosomes (indicated by arrows) in Rpa1cKO metaphase I spermatocytes. (D) Quantification of chiasmata from control and Rpa1cKO metaphase I cells. n, number of cells counted from three experiments. (E) TUNEL analysis of stage XII seminiferous tubules from adult control and Rpa1cKO males. Note the TUNEL-positive (green) metaphase I spermatocytes in the Rpa1cKO tubule. Scale bar, 25 μm. (F) Quantification of apoptotic cells of stage XII tubules from adult control and Rpa1cKO males. n, number of stage XII tubules from two experiments. ns, no significance; ***, p ≤ 0.001 (Student’s t-Test); dpt, days post tamoxifen injection. (G) Dual functions of RPA in meiotic recombination. The upper diagram illustrates the distribution of recombination proteins and timing of key recombination events during male meiosis. The lower diagram depicts the defects in the absence of RPA: depletion of RAD51/DMC1, reduction in MLH1 foci, and two rounds of apoptosis (the first round at stage IV and the second round at stage XII). Abbreviations: Lep, leptotene spermatocytes; Zyg, zygotene; Pac, pachytene; Dip, diplotene; Met I, metaphase I; Ana I, anaphase I.