Abstract
Senile plaques and neurofibrillary tangles are the principal histopathologic hallmarks of Alzheimer disease. The essential constituents of these lesions are structurally abnormal variants of normally generated proteins: Aβ protein in plaques and tau protein in tangles. At the molecular level, both proteins in a pathogenic state share key properties with classic prions, i.e., they consist of alternatively folded, β-sheet-rich forms of the proteins that autopropagate by the seeded corruption and self-assembly of like proteins. Other similarities with prions include the ability to manifest as polymorphic and polyfunctional strains, resistance to chemical and enzymatic destruction, and the ability to spread within the brain and from the periphery to the brain. In AD, current evidence indicates that the pathogenic cascade follows from the endogenous, sequential corruption of Aβ and then tau. Therapeutic options include reducing the production or multimerization of the proteins, uncoupling the Aβ-tauopathy connection, or promoting the inactivation or removal of anomalous assemblies from the brain. Although aberrant Aβ appears to be the prime mover of AD pathogenesis, once set in motion by Aβ, the prion-like propagation of tauopathy may proceed independently of Aβ; if so, Aβ might be solely targeted as an early preventive measure, but optimal treatment of Alzheimer disease at later stages of the cascade will require intervention in both pathways.
Keywords: Abeta, Alzheimer, amyloid, aging, dementia, neurodegeneration, prion, proteopathy, seeding, tau
Alzheimer disease
Epidemiology, signs and symptoms.
One of the most feared hazards of growing old is the profound deterioration of mental faculties known as dementia. More than 50 different conditions are associated with dementia (Vonsattel and Hedley-White, 2001), but of these, Alzheimer disease (AD) is the most common, with a world-wide prevalence in 2010 of approximately 35 million people (Dartigues, 2009; Holtzman et al., 2011; Reitz et al., 2011). The incidence and prevalence of AD double every 5 years between the ages of 65 and 95 (Kawas and Katzman, 1999). As the average life expectancy of populations grows in many parts of the world, and in the absence of an effective preventive or treatment, as many as 115 million people are expected to have AD in the year 2050 (Dartigues, 2009). The social and economic costs of the disease will rise accordingly (Wimo et al., 2013), with an ever greater burden of caring for afflicted persons falling on younger generations. Disease-modifying treatments are urgently needed, but these can only emerge from a deep understanding of Alzheimer disease itself. A defining pathologic feature of AD is the abnormal accumulation in the brain of two proteins - Aβ and tau; recent evidence shows that this process is initiated and sustained by a prion-like mechanism of seeded protein aggregation.
AD typically begins with the gradual onset of mild cognitive impairment, progressing inexorably to dementia with an average clinical duration of 7–10 years (Holtzman et al., 2011) (although the timecourse is variable [see Chapter 24]). The signs and symptoms shown by individual patients also can vary substantially, but the diagnosis of AD is established by the universal presence of core attributes, specifically progressive dementia in the context of characteristic lesions in the brain: senile (Aβ) plaques and neurofibrillary (tau) tangles.
Dementia can be defined as “a decline from a person’s previously established level of intellectual function that is sufficient to interfere with the everyday performance of that individual” (Holtzman et al., 2011). Based on the criteria set forth in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) dementia due to AD is defined as “the insidious onset and gradual progression of substantial impairment in learning and memory and at least one other cognitive domain (complex attention, executive function, language, perceptual-motor, or social cognition) that interferes with independence in everyday activities” (Walker and Jucker, 2017). An important feature of these definitions is that the impairments are substantial, and thus become incapacitating even under ordinary circumstances.
Differential diagnosis and biomarkers.
Until recently, senile plaques and neurofibrillary tangles could only be identified by microscopic analysis of brain samples, but increasingly sensitive and specific diagnostic tests are emerging that enable the detection of proteopathic abnormalities in living subjects. These include radiolabeled imaging agents for Aβ and tau in the brain, and assays for quantitation of the proteins in CSF (Lewczuk et al., 2015; Olsson et al., 2016; Villemagne et al., 2017). Investigations of these biomarkers indicate that the disease process begins two decades or more before the onset of demonstrable cognitive impairment (Jack et al., 2010; Jack and Holtzman, 2013). In addition, the presence of genetic risk factors such as the ε4 allele of apolipoprotein E (APOEε4) can reinforce the in-life diagnosis of AD.
It is important to place AD in the context of other brain changes that impair intellectual capacities in the elderly. In younger patients with autosomal dominant causes of AD (below), the disease is relatively unambiguous histopathologically, i.e., lesions other than plaques and tangles are rare. With advancing age, additional disorders are increasingly likely to contribute to dementia, including cerebrovascular disease, hippocampal sclerosis, and such cerebral proteopathies as α-synucleinopathy, TDP-43 proteopathy, and others (Vonsattel and Hedley-White, 2001; Nelson et al., 2012). These maladies can cause dementia on their own, but they also sometimes co-exist with AD, complicating diagnosis, exacerbating the clinical course, and likely diminishing the effectiveness of treatments directed at only one of the conditions. In addition, potentially reversible causes of a dementia-like state must be ruled out, such as depression, infections, drugs and drug interactions, thyroid dysfunction, tumors, and vitamin B12 deficiency, as intervention in these instances might at least partially restore cognitive function (Tripathi and Vibha, 2009; Holtzman et al., 2011).
Genetics.
The probability of developing AD is influenced by certain genetic risk factors, which include rare causative, autosomal dominant mutations with essentially complete penetrance, as well as diverse genetic polymorphisms that modulate risk to varying degrees (Tanzi, 2012; Wingo et al., 2012). Autosomal dominant mutations associated with AD all occur in the genes that code for the Aβ-precursor protein (APP) or for presenilin-1 or presenilin-2 (the presenilins being key components of intramembranous protease complexes that liberate Aβ from APP (Hardy, 2006; Holtzman et al., 2011)). The gene encoding APP is on chromosome 21, and the genes encoding presenilin-1 and presenilin-2 are on chromosomes 14 and 1, respectively.
AD-linked mutations in the presenilins and in the APP regions flanking Aβ alter the processing of APP, but mutations within the Aβ segment of APP often modify its potential to aggregate and its tissue-specificity (Holtzman et al., 2011; Haass et al., 2012). An unusual variant in the APP gene that results in an alanine to threonine substitution at position 2 of Aβ reduces the production (Jonsson et al., 2012) and aggregation proclivity (Benilova et al., 2014) of Aβ, thereby lowering the risk of AD (Jonsson et al., 2012). In contrast, an alanine to valine replacement at this position increases the production and aggregability of Aβ, causing an autosomal recessive type of AD (Di Fede et al., 2009) with unusual neuropathologic features (Giaccone et al., 2010). Furthermore, because most patients with Down syndrome (trisomy 21) have an extra copy of the APP gene on chromosome 21, they are at greatly increased risk of developing AD as they age (Head et al., 2016). Together, these genetic data consistently implicate Aβ in the ontogeny of heredofamilial forms of AD.
Dominant and recessive genetic causes account for less than 1% of all AD cases (Holtzman et al., 2011). How, then, are genetics linked to idiopathic AD? A study of twins in Sweden indicated that the heritability for AD is as high as 79% (Gatz et al., 2006), although most of the implicated polymorphisms individually have only a slight influence on risk (Humphries and Kohli, 2014). An exception is the gene that encodes apolipoprotein E (ApoE) (Yu et al., 2014), a protein that mediates lipid transport throughout the body and is the major apolipoprotein in the brain (Chouraki and Seshadri, 2014). The three major protein isoforms of ApoE in human populations are ApoE2, ApoE3 and ApoE4. The most frequent isoform is ApoE3 (~78%), followed by ApoE4 (~14%) and ApoE2 (~8%) (Liu et al., 2013). Bearers of the APOEε4 allele have an allele-dose-dependent increase in the risk of AD, with heterozygotes having a 2- to 5-fold increase in risk, and homozygotes a 12- to 15-fold increase (Chouraki and Seshadri, 2014). The mechanism by which APOEε4 predisposes carriers to AD is probably multifaceted (Potter and Wisniewski, 2012; Yu et al., 2014; Huang et al., 2017), but it is known that bearers of APOEε4 begin to accumulate Aβ in the brain at least a decade earlier in life than do non-bearers (Warzok et al., 1998; Walker et al., 2000; Resnick et al., 2015). Thus, APOEε4, like the known dominant and recessive genetic risk factors (above), appears to augment the probability of developing AD by advancing the onset of the Aβ cascade. Indeed, all known AD-linked mutations affect the production, removal, trafficking, or tendency to aggregate of Aβ (Hardy and Selkoe, 2002).
Other risk factors.
In addition to the genetic risk factors for AD, numerous environmental and endogenous risk factors have been identified. These include advancing age, traumatic brain injury, diabetes and metabolic disorders, inflammation, vascular disorders, gender, and lifestyle (Holtzman et al., 2011; Killin et al., 2016; Lafortune et al., 2016; Pike, 2017). In some instances these should be considered as risk factors for dementia, broadly defined, rather than for AD per se. For example, multiple small infarcts might raise the likelihood of dementia independently of AD, or they may advance the onset of dementia in people who also are incubating AD pathology in the brain.
The neuropathology of AD in the context of the prion paradigm
Amyloid.
As in the case of most proteopathies (including the prionoses), the proteins that are implicated in the development of AD are structurally abnormal manifestations of proteins that are normally generated by cells. The abnormalities often involve an altered 3-dimensional architecture (misfolding), which can be promoted by amino acid substitutions, post-translational modifications, sequence expansions or truncations, and such characteristics of the local milieu as temperature and pH (Eisenberg and Jucker, 2012). In addition, factors that increase the concentration of certain proteins (e.g., by raising their production or impairing their removal/degradation) can elevate the risk of disease (Jucker and Walker, 2013).
A frequent indication that a protein is structurally corrupted at the molecular level is its enhanced tendency to form amyloid. In general, amyloid is a state in which a protein accumulates in tissues as masses of ~10nm-diameter fibrils with a characteristic cross-β X-ray diffraction pattern and cross-polarization-induced birefringence after staining with the dye Congo red (Sipe et al., 2016), indicative of increased β-sheet molecular structure (Eisenberg and Jucker, 2012). In some biological circumstances the amyloid state is functionally advantageous, particularly in prokaryotes (Fowler et al., 2007; Greenwald and Riek, 2010) (amyloid-like fibrils consisting of stacked, cross-α helices have been identified in the bacterium Staphylococcus aureus(Tayeb-Fligelman et al., 2017), but α-helix-based amyloid has not been described in eukaryotes). In mammals, amyloid is often pathogenic; more than 30 different amyloidoses have been reported (Sipe et al., 2016), many of which occur outside the central nervous system (Westermark et al., 2017).
Within the brain, it is not uncommon to find some degree of Aβ-amyloidosis and tauopathy in the elderly; in those with dementia, abundant Aβ plaques and tau tangles are the two types of amyloid that are pathognomonic for AD (Holtzman et al., 2011; Nelson et al., 2012) (Figure 1). The formation of amyloid by Aβ and tau is an obvious sign of a proteopathic process, but small oligomeric assemblies may actually be the more toxic form of the proteins (Lambert et al., 1998; Haass and Selkoe, 2007; Gerson and Kayed, 2016; Yang et al., 2017). Aβ in the amyloid state is virtually always present in AD, but an instructive exception is a rare hereditary type of AD caused by a mutation that changes glutamate to glycine at position 22 of Aβ (E22G; the ‘arctic’ mutation). This mutation results in early-onset AD in which Aβ plaques lack the prototypical amyloid cores (Kalimo et al., 2013), indicating that ‘amyloid’ in the strict sense is not required to drive the Aβ-cascade. Similarly, even though misfolded prion protein (PrP) has an enhanced ability to form amyloid, PrP-amyloid per se is not obligatory for the expression of prion disease (DeArmond and Prusiner, 1995).
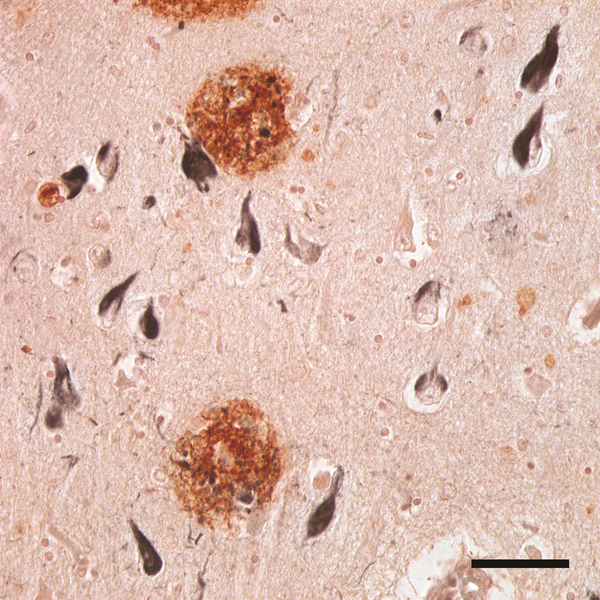
Figure 1.
The canonical neuropathologic features of AD include senile (Aβ) plaques (reddish brown) and neurofibrillary (tau) tangles (black). Aβ was immunostained with rabbit polyclonal antibody R398 to Aβ42, and tau was immunostained with mouse monoclonal antibody MC1 to paired helical filaments. CA1 field of the hippocampus. Bar = 50μm.
This point bears emphasis because of the unproductive controversy (Drachman, 2014) that still bedevils the “amyloid cascade hypothesis” (aka the “amyloid hypothesis”) of AD (Hardy and Selkoe, 2002; Selkoe and Hardy, 2016). The ‘amyloid’ in the original formulation of this concept refers to β-amyloid (i.e., Aβ in a β-sheet-rich, polymerized state). There can be little doubt that Aβ is a driving force in the genesis of AD (Jack et al., 2010; Selkoe, 2011; Bateman et al., 2012; Walker and Jucker, 2015), or that β-amyloid accumulation per se is detrimental to cognition, particularly when embodied as neuritic plaques (Nelson et al., 2012). However, tauopathy is an essential downstream consequence that correlates more strongly with the degree of dementia than does the number of Aβ plaques (Wilcock and Esiri, 1982; Crystal et al., 1988; Bierer et al., 1995). This apparent inconsistency mainly reflects the importance of tauopathy for the clinical expression of the disease, but it does not invalidate the instigating role of multimeric Aβ in AD. A review of published work by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) concluded that “CSF biomarkers are consistent with disease trajectories predicted by β-amyloid cascade … and tau-mediated neurodegeneration hypotheses for AD” (Weiner et al., 2013). Because tau and Aβ both self-aggregate by the templated corruption of like proteins by misfolded seeds, once tauopathy is set in motion, it is possible that the two proteopathies progress along separate paths, both temporally and spatially.
The tau protein normally is involved in the stabilization of cellular microtubules (Spillantini and Goedert, 2013). In AD and other tauopathies, tau misfolds and becomes hyperphosphorylated; like Aβ, the altered tau molecules aggregate to form soluble oligomers and long, β-sheet-rich polymers that have the characteristics that define amyloid. The tau fibrils bundle together as neurofibrillary tangles in neurons (Figure 1), although tauopathy also can afflict glial cells (Kovacs, 2015).
Tauopathy occurs in association with many brain disorders besides AD (Nelson et al., 2012). The primary tauopathies are disorders in which tau aggregation is the major abnormality (Spillantini and Goedert, 2013; Crary et al., 2014; Kovacs, 2015); in many conditions however, tauopathy is secondary to various types of injury or stress to the brain (Nelson et al., 2012). In human prion diseases, tauopathy is variable in appearance and degree (DeArmond et al., 2004; Kovacs et al., 2016b), and in instances where it is relatively prominent (such as Gerstmann-Sträussler-Scheinker disease and variant Creutzfeldt-Jakob disease [vCJD]) the cytology and anatomic distribution often differ from those seen in AD (Giaccone et al., 2008; Kovacs et al., 2016b).
The extended neuropathology of AD.
In addition to the canonical lesions that define AD – Aβ plaques and tau tangles – other changes are present in the brain that complicate the disease phenotype. One is the accumulation of Aβ in and around the walls of cerebral blood vessels, a condition known as Aβ-type cerebral amyloid angiopathy (Aβ-CAA). Aβ-CAA weakens the vascular wall and elevates the risk of intracranial hemorrhage (Biffi and Greenberg, 2011). Like Aβ plaques and tauopathy, Aβ-CAA is not specific to AD, and its prevalence increases with advancing age (Revesz et al., 2003; Biffi and Greenberg, 2011). However, some degree of Aβ-CAA is almost always present in AD (Attems and Jellinger, 2014; Vinters, 2015; Kapasi and Schneider, 2016), and it is severe in around 25% of cases (Charidimou et al., 2012). The factors that drive the inconsistent occurrence of Aβ-CAA in different people remain uncertain.
Other alterations are found to variable extents among end-stage AD cases, and most of these anomalies lack diagnostic specificity for the disease. Macroscopically, loss of brain tissue and concomitant expansion of the ventricles are common, but this varies among regions and among patients (Hauw and Duyckaerts, 2001). Evidence of inflammation includes reactive microglia and astrocytes, especially in association with Aβ plaques, as well as increased inflammatory mediators such as cytokines (Duyckaerts et al., 2009). Granulovacuolar degeneration, perisomatic granules and Hirano bodies may be present (Duyckaerts et al., 2009), but their significance for AD per se is uncertain. Many different neuronal systems are compromised in AD (Mann and Yates, 1986; Hauw and Duyckaerts, 2001), some more markedly than others, and synapses are regionally depleted (Terry et al., 1999; Duyckaerts et al., 2009). In some cases of AD, spongiform change is evident that, though generally less severe, can resemble that seen in CJD (Smith et al., 1987; Sherzai et al., 2013) (Figure 2). As noted above, other neurodegenerative conditions might be present in the brain along with the lesions of AD, particularly in older patients.
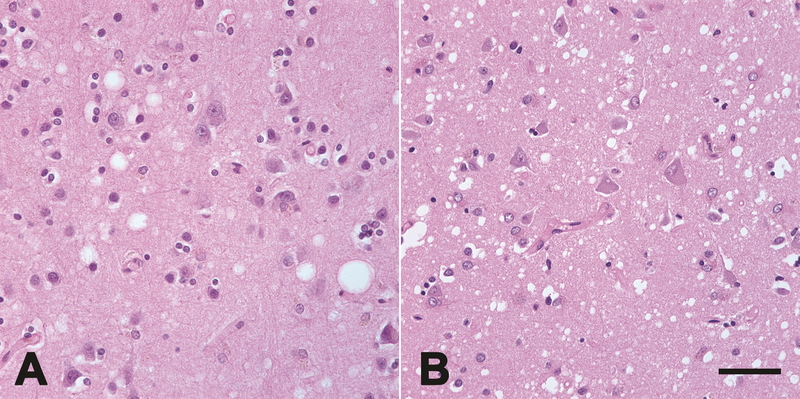
Figure 2.
Spongiform change (vacuoles, seen in these micrographs as white holes) in the neocortex of an AD patient (A) and in a patient with CJD (B). Spongiform change is not unique to prion diseases, but it is less common in AD, and when it occurs it is generally mild (Smith et al., 1987; Sherzai et al., 2013). Hematoxylin and eosin stain. Bar = 50μm.
Regardless of the complexity of damage to the brain, the essential and unifying feature of AD is the obligatory presence of aggregated Aβ and tau proteins. For this reason, extensive research has been directed toward determining how the proteins misfold, self-assemble, and propagate their pathogenic features, a process that shares important commonalities with the molecular pathogenesis of prion diseases (Walker and LeVine, 2000; Walker et al., 2006; Jucker and Walker, 2013; Prusiner, 2013; Goedert, 2015; Walker and Jucker, 2015; Walker et al., 2016).
The prion-like properties of aggregated Aβ
The idea that Alzheimer disease might arise by a pathogenic mechanism similar to that of prion diseases has a fairly long history (Farquhar and Gajdusek, 1981; Prusiner, 1984). Based on their success in transmitting kuru and Creutzfeldt-Jakob disease to nonhuman primates (Gajdusek et al., 1966; Gajdusek et al., 1968; Gibbs et al., 1968), and on the hypothesis that a ‘slow virus’ might be involved in other neurodegenerative disorders (Gajdusek, 1977), D. Carleton Gajdusek’s group attempted to experimentally transmit AD to several species of nonhuman primates via intracerebral injection of AD brain homogenates. They tentatively reported that the attempt was unsuccessful (Goudsmit et al., 1980). In Great Britain, Ridley and Baker undertook similar transmission experiments in marmosets (Callithrix jacchus). After an incubation period of more than 5 years, they detected a significant increase in the senile plaque load of the host animals (Baker et al., 1994). The causative agent, however, remained uncertain.
When transgenic mouse models expressing human-type APP became available, experiments were initiated to explicitly test the hypothesis that Aβ can be induced to aggregate in the living brain by a prion-like mechanism (Kane et al., 2000). These studies showed that Aβ plaques and CAA are seedable by brain extracts from AD patients, but not by extracts derived from control brains that were devoid of aggregated Aβ (Kane et al., 2000; Walker et al., 2002; Meyer-Luehmann et al., 2006) (Figure 3). AD brain extracts infused into the brains of wild-type mice (which express an aggregation-resistant sequence of Aβ) did not yield Aβ deposits.
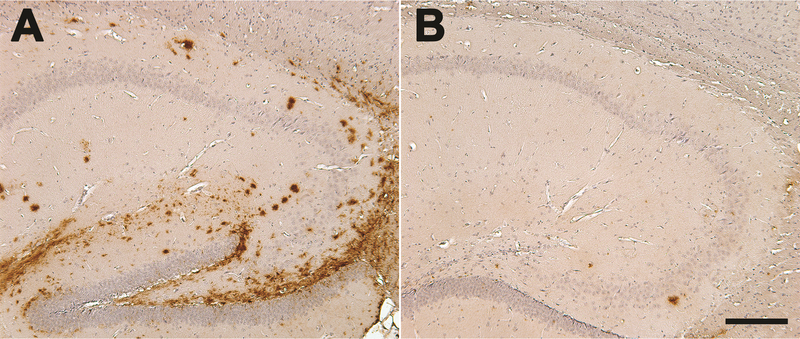
Figure 3.
Seeded Aβ deposition (brown) in the hippocampus of an 8-month-old TG2576 APP-transgenic mouse (sagittal sections; rostral is to the right). The hippocampus of one hemisphere (A) was injected 5 months earlier with clarified cortical extract from an AD case, and the contralateral hippocampus (B) received a similar amount of control brain extract lacking aggregated Aβ. The induced deposits emerge histologically in this model after around 2–3 months, and increase thereafter. Sections were incubated with polyclonal antibody R398 to Aβ42. Hematoxylin counterstain (blue). Bar = 200μm.
Subsequent experiments showed unequivocally that the active agent is aggregated Aβ, and that the ability of Aβ to seed as well as the characteristics of the resulting deposits are governed by both the agent and the host (Meyer-Luehmann et al., 2006). These findings have been confirmed and extended by other laboratories (Watts et al., 2011; Morales et al., 2012; Stohr et al., 2012; Duran-Aniotz et al., 2013; Stohr et al., 2014; Duran-Aniotz et al., 2014; Watts et al., 2014; Morales et al., 2015a; Burwinkel et al., 2018), and the collective experiments have established that the molecular features of Aβ seeds are essentially the same as those that define the pathogenicity of prions (Jucker and Walker, 2013; Morales et al., 2015b; Walker and Jucker, 2015; Walker et al., 2016). Key commonalities between Aβ seeds and PrP-prions are summarized as follows:
The active seeding agent is a form of the protein itself. In addition to brain extracts from AD patients, extracts from APP-transgenic mice (Meyer-Luehmann et al., 2006) and aged monkeys (Rosen et al., 2016) can seed Aβ deposition as long as aggregated Aβ is present in the donor brain. The degree of Aβ-seeding is directly related to the concentration of Aβ in the brain extract (Meyer-Luehmann et al., 2006; Fritschi et al., 2014b), and even extremely small amounts of Aβ seeds are capable of stimulating aggregation in the brain (Fritschi et al., 2014b; Morales et al., 2015a). Immunodepletion of Aβ from the donor extract prior to injection nullifies the seeding effect (Meyer-Luehmann et al., 2006; Duran-Aniotz et al., 2014). Synthetic, pre-aggregated Aβ is capable of seeding deposition in vivo (Stohr et al., 2012), albeit relatively weakly (see below).
Aβ seeds are rich in β-sheet secondary structure. Amyloid fibrils of all types, including Aβ-amyloid and PrP-amyloid, are rich in β-sheets in which the individual β-strands run approximately perpendicular to the long axis of the fibrils (Eisenberg and Jucker, 2012). In vitro studies by Lansbury and colleagues demonstrated that pre-aggregated, β-sheet-rich seeds of synthetic Aβ efficiently induce monomeric Aβ to acquire β-sheet and assemble into amyloid (Harper and Lansbury, 1997). In addition, in vivo seeding experiments have shown that denaturation of Aβ-seed-rich brain extracts with formic acid (which disrupts the 3-dimensional architecture of proteins) negates the ability of the extracts to induce plaque formation (Meyer-Luehmann et al., 2006).
-
Misfolded Aβ can manifest as structurally and functionally variant strains. In the canonical (PrP) prion diseases, prion traits and the host response vary in ways that suggest alternative structural and functional ‘strains’ of the agent. Strain differences, in turn, have been linked to dissimilarities in PrP amino acid sequence, protease sensitivity, resistance to denaturants, and glycosylation patterns (McKintosh et al., 2003; Weissmann, 2004; Wiseman et al., 2015). However, a critical factor governing prion infectivity and disease phenotype is the molecular conformation of pathogenic PrP (PrPTSE, or PrPSc]) (Peretz et al., 2002; Tanaka et al., 2006; Gambetti et al., 2011). Distinct strains of PrPSc often yield characteristic patterns of lesion structure and distribution in the brain (Peretz et al., 2002; DeArmond et al., 2004). An important indication that variant CJD (the human prionosis that is linked to bovine spongiform encephalopathy) is caused by a novel prion strain was the discovery of atypical lesions termed florid plaques in affected humans (Ironside et al., 2000).
As in the case of PrP-prions, Aβ can fold into strain-like variants both in vitro (Petkova et al., 2005; Nilsson et al., 2007; Yagi et al., 2007; Paravastu et al., 2008; Meinhardt et al., 2009; Miller et al., 2010; Kodali et al., 2010; Agopian and Guo, 2012; Spirig et al., 2014; Tycko, 2015; Tycko, 2016) and in vivo (Meyer-Luehmann et al., 2006; Rosen et al., 2010; Rosen et al., 2011; Lu et al., 2013; Heilbronner et al., 2013; Watts et al., 2014; Stohr et al., 2014; Cohen et al., 2015; Condello et al., 2018; Rasmussen et al., 2017). Cerebral Aβ assemblies in humans with AD vary in terms of plaque morphology (Wisniewski et al., 1989; Thal et al., 2006), ligand binding characteristics (Rosen et al., 2010; Condello et al., 2018; Rasmussen et al., 2017), solid-state nuclear magnetic resonance features (Qiang et al., 2017), as well as conformational stability and other biophysical characteristics (Cohen et al., 2015). Interestingly, Aβ extracted from the autopsied brains of nondemented elderly subjects exhibits molecular-level features that differ in some ways from AD-derived Aβ (Piccini et al., 2005; Portelius et al., 2015). Whether these differences are indicative of fundamentally distinctive strains of Aβ, or whether they reflect early versus late stages in the pathogenesis of AD is not certain.
Experimental studies in transgenic mice have shown that strain-like features of aggregated Aβ can be transferred from donor to host by exogenous seeding. Specifically, in the absence of seeding, APP23 mice and APP/PS1 mice develop Aβ plaques with different morphologies and ratios of the 40- and 42-amino acid lengths of Aβ (Aβ40 and Aβ42); when Aβ seeds from one transgenic mouse model were infused intracerebrally into the other, the plaque morphology (Meyer-Luehmann et al., 2006), spectral signature of bound conformation-sensitive thiophene ligands, and the Aβ40:42 ratio (Heilbronner et al., 2013) were influenced both by the source of the seeds and the type of murine host. In addition, strain-like features of Aβ from human AD cases can be at least partially replicated in mouse models (Condello et al., 2018; Rasmussen et al., 2017).
Aβ seeds vary in size and sensitivity to proteinase K. Infectious PrP-prions exist in a wide range of sizes, the most potent of which are small and soluble (Silveira et al., 2005). Similarly, Aβ seeds can range from large fibrils to small, oligomeric seeds with high biologic potency (Langer et al., 2011). Large Aβ seeds are relatively resistant to inactivation by proteinase K, whereas - like PrP-prions - oligomeric Aβ seeds are readily inactivated by the enzyme (Langer et al., 2011).
Some Aβ seeds are durable. When Aβ-rich brain extracts are boiled for 5 minutes prior to infusion into host mice, a significant fraction of bioactive Aβ seeds remain (Meyer-Luehmann et al., 2006). In addition, similar to PrP-prions, Aβ seeds retain their potency in donor brain tissue that has been in formaldehyde for years (Fritschi et al., 2014a). Aβ seeds also are durable within the living brain; they retain some bioactivity (albeit with progressively diminishing potency) for at least 6-months after infusion into the brains of mice engineered to lack APP, and which therefore are incapable of replicating Aβ in any form (Ye et al., 2015a). Analogously, PrP-prions have been reported to persist in the brains of PrP-deficient mice for up to 600 days (Diack et al., 2016). Likewise, AA amyloidosis in systemic organs can be promoted by fibrillar AA seeds (‘amyloid enhancing factor’) that persist in mice for at least 6 months (Lundmark et al., 2002). The endurance of some proteopathic seeds may result from their ability to adopt the highly stable, β-sheet-rich amyloid state (above).
-
Aβ seeds spread systematically within the brain. As with PrP-prions (Fraser, 1982; Buyukmihci et al., 1983; Kimberlin and Walker, 1986; Liberski et al., 2012; Rangel et al., 2014) and other proteopathic seeds (Clavaguera et al., 2009; Clavaguera et al., 2013; Ahmed et al., 2014; Iba et al., 2015; Boluda et al., 2015; Rey et al., 2016; Hock and Polymenidou, 2016)), Aβ seeds introduced into one part of the brain induce protein aggregation that spreads systematically to interconnected regions (Hamaguchi et al., 2012; Ye et al., 2015b). In APP-transgenic mouse models, Aβ seeds injected into the peritoneal cavity (Eisele et al., 2010; Eisele et al., 2014) or intravenously (Burwinkel et al., 2018) travel to the brain, where many of the induced deposits are associated with cerebral blood vessels.
The cellular mechanisms involved in the trafficking of Aβ seeds remain uncertain. Extracellular, soluble Aβ is taken up by cultured cells and concentrated in the acidic environment of endosomes/lysosomes, where the Aβ assembles into higher molecular weight seeds (Hu et al., 2009). Oligomeric Aβ seeds have been described that are bound to intracellular membranes and that strongly stimulate Aβ aggregation in vitro and in vivo (Marzesco et al., 2016). In cell culture experiments, Aβ seeds were demonstrated to spread by transfer from neuron to neuron (Nath et al., 2012; Domert et al., 2014), and neuroanatomical patterns of deposition are consistent with spread along neuronal pathways (Hamaguchi et al., 2012; Ronnback et al., 2012; Ye et al., 2015b) by active cellular transport and/or diffusion (Eisele and Duyckaerts, 2016). In addition, there is evidence that macrophages can phagocytose and translocate Aβ seeds (Eisele et al., 2014; Cintron et al., 2015).
Small, cell-derived extracellular vesicles such as exosomes have been linked to the transmissibility of PrP-prions (Fevrier et al., 2004; Properzi et al., 2015; Guo et al., 2016a). Extracellular vesicles also have been suggested to ferry Aβ between cells (Rajendran et al., 2006), although their influence on the pathogenesis of AD – positive or negative - remains uncertain (Joshi et al., 2015).
Aβ aggregation can be instigated de novo. PrP-prions induce prion disease in animals that are unlikely to have acquired the disease without exposure to exogenous prions. In contrast, many of the experiments showing seeding of Aβ have been undertaken in transgenic mouse models that, with age, eventually develop Aβ-plaques and CAA spontaneously. To determine whether Aβ deposition can be seeded in normally resistant animals, Aβ-seed-rich brain extracts were injected intracerebrally into transgenic rodent models that do not generate Aβ lesions within their average lifespans; these studies indicate that Aβ deposition is inducible de novo, and in this paradigm is not simply an acceleration of an ongoing process (Morales et al., 2012; Rosen et al., 2012).
Aβ proteopathy is serially transmissible. Similar to PrP-prions, different strains of Aβ seeds can be successively transmitted from the initially seeded mice to subsequent hosts (Watts et al., 2014).
Prion-like properties of Aβ: Open questions
Pure, pre-aggregated synthetic Aβ is able to seed deposition in vivo, but synthetic Aβ seeds are much weaker than are Aβ seeds derived from the brain (Stohr et al., 2012). Correspondingly, generating infectious prions from purified, recombinant PrP has long been a challenge (Legname et al., 2004). Both Aβ seeds and PrP-prions thus are most potent when they are generated within living tissues. The infectivity of recombinant PrP-prions can be augmented by adding certain cofactors to the medium during aggregation (Wang et al., 2010; Deleault et al., 2012; Zhang et al., 2014). It is possible that specific cofactors also are required to optimize the bioactivity of Aβ seeds in vivo. The lipid environment, for instance, influences the pathobiology of Aβ (Morgado and Garvey, 2015), and lipids are essential for the high-affinity binding of the β-amyloid imaging agent Pittsburgh Compound B (PiB) to cerebral Aβ (Matveev et al., 2014). Potent, in vivo–active Aβ seeds recently have been generated by the seeded conversion of synthetic Aβ in a hippocampal slice culture model (Novotny et al., 2016). Clarifying the conditions that influence protein aggregation, seeding, and toxicity in living systems could disclose new therapeutic objectives for multiple proteopathies. Insights might also emerge from an analysis of senescent nonhuman primates which, despite substantial accumulation of human-sequence Aβ with age, exhibit neither significant tauopathy nor dementia (Rosen et al., 2016). A possible parallel in the prion field is the dissociation of PrP-amyloid seeding and transmission of spongiform encephalopathy in a mouse model (Piccardo et al., 2013).
Another open question is why the Aβ that is present in the CSF of AD patients only weakly seeds the aggregation of synthetic Aβ in vitro, and fails to seed deposition in the brains of APP-transgenic mice even at Aβ concentrations that exceed the levels in brain extracts by a factor of ten (Fritschi et al., 2014b). The reasons for the poor seeding efficiency of CSF Aβ are unknown, but the Aβ assemblies in CSF were found to be smaller and mostly devoid of N-terminally truncated variants compared to brain-derived Aβ (Fritschi et al., 2014b). Additionally, other substances in the CSF, such as cystatin C (Kaeser et al., 2007), might interfere with the seeding capacity of multimeric Aβ.
Finally, in an intriguing intersection of disease-related proteins, the normal, cellular form of the prion protein (PrPC) was discovered to be a cell-surface receptor for oligomeric Aβ (Salazar and Strittmatter, 2016). The implications of the Aβ-PrP interaction for AD appear to be complex, as its impact on Aβ toxicity or aggregation may be either deleterious (Um et al., 2012; Rushworth et al., 2013; Hu et al., 2014; Lauren, 2014) or beneficial (when it occurs in extracellular vesicles (Falker et al., 2016) or between Aβ and soluble (glycophosphatidylinositol anchor-free) PrP (Nieznanski et al., 2012)).
The prion-like properties of aggregated tau
At the ultrastructural level, neurofibrillary tangles in AD consist predominantly of characteristic paired helical filaments (Crowther, 1991) that result from the ectopic polymerization of hyperphosphorylated tau protein (Lee et al., 2001; Spillantini and Goedert, 2013). Tau hyperphosphorylation is thought to be an early stage in the formation of tangles (although it can occur as a reversible phenomenon under such conditions as fetal development, hibernation, and hypothermia (Spillantini and Goedert, 2013)).
Considerable evidence now supports the inclusion of tauopathy among the disorders that share a prion-like mechanism of pathogenesis. Similar to in vivo Aβ seeding, the accumulation of hyperphosphorylated tau is inducible in the brains of tau-transgenic host mice by infusion of aggregated tau seeds (Clavaguera et al., 2009; Guo and Lee, 2011; Holmes et al., 2014; Clavaguera et al., 2015; Peeraer et al., 2015; Polanco et al., 2016; Takeda et al., 2016; Gerson et al., 2016). The ensuing tauopathy spreads systematically from the site of injection to axonally connected regions of the brain (Clavaguera et al., 2009; Clavaguera et al., 2013; Ahmed et al., 2014; Stancu et al., 2015; Narasimhan et al., 2017), consistent with the uptake, transport, and discharge of tau seeds by neurons (Frost et al., 2009; Wu et al., 2013; Sanders et al., 2014). Neuronal activity augments the release of tau from cells in vitro, and also increases the amount of tauopathy in vivo (Wu et al., 2016). Additionally, tau antisense oligonucleotides decrease tau expression and pathology in mouse models, and also reverse pathologic tau seeding (DeVos et al., 2017). Like Aβ-proteopathy and prion disease, tauopathy can be induced in the brain by tau seeds that have been infused into the peritoneal cavity (Clavaguera et al., 2014), and bioactive tau seeds exist in a range of sizes (Lasagna-Reeves et al., 2012; Mirbaha et al., 2015; Gerson et al., 2016; Jackson et al., 2016).
Brain extracts from donors with clinicopathologically distinct human tauopathies induce tau lesions in host mice that resemble the lesions in the corresponding human disorders (Clavaguera et al., 2013; Sanders et al., 2014; Boluda et al., 2015; Narasimhan et al., 2017), indicating that tau, like Aβ and PrP, can misfold into replicable proteopathic strains (Sanders et al., 2014). At the cellular level, multimeric tau is taken up by a heparan sulfate proteoglycan-associated mechanism (Holmes et al., 2013), and the aggregates enter cells via macropinocytosis (Holmes et al., 2013; Falcon et al., 2015). Tau strains instigate distinct regional and cellular patterns of inclusions, and the strains can be reliably propagated in cell cultures (Kaufman et al., 2016). The bioactivity of tau strains in HEK cells was shown to be governed by the isoform composition (3-repeat and/or 4-repeat) of the tau seeds along with the isoform expression by the host cells (Woerman et al., 2016). Tau seeds are present in the human brain early in the development of tauopathy, and possibly prior to the histologic appearance of hyperphosphorylated tau within neurons (Furman et al., 2017).
Tau seeding differs from Aβ seeding in that tauopathy is readily inducible by AD brain extracts in non-transgenic (wild-type) mice (Audouard et al., 2016; Guo et al., 2016b). In addition, recombinant tau fibrils can fairly efficiently instigate tauopathy in tau-transgenic mice (Lasagna-Reeves et al., 2012; Clavaguera et al., 2013; Iba et al., 2013; Peeraer et al., 2015), although the potency of recombinant tau is less than that of tau that originates in brain samples (Falcon et al., 2015). Recombinant tau fibrils did not seed tauopathy in wild-type mice, possibly due to distinct conformational differences between artificially assembled fibrils and those generated in the brain (Guo et al., 2016b).
In the CSF of AD patients and transgenic mice expressing human-type tau, seed-competent tau is present that can stimulate tauopathy in cultured cells (Takeda et al., 2016), and some tau seeds in AD CSF appear to be associated with extracellular vesicles (Wang et al., 2017). The seeding capability of CSF tau in vivo, however, has not been reported; because CSF Aβ does not readily seed plaques or CAA in APP-transgenic mice (Fritschi et al., 2014b) (see above), a similar analysis of in vivo tau seeding by CSF from patients with AD (and other tauopathies) in the appropriate models could be informative.
These experiments collectively underscore the prion-like molecular properties of aggregated tau, but current evidence indicates that tauopathy, like Aβ-proteopathy, is not infectious in the customary sense of being facilely transmissible from one organism to another (Walker and Jucker, 2015). Rather, in AD, the process of tau misfolding and propagation takes place entirely within the affected organism. To model the endogenous emergence and spread of tauopathy, genetically modified mice were studied in which the expression of a disease-associated human tau transgene is restricted principally to projection neurons of the entorhinal cortex (de Calignon et al., 2012; Liu et al., 2012). The mice developed tauopathy initially in the entorhinal cortex, as expected, but with passing time the abnormalities successively emerged in axonally connected brain areas (de Calignon et al., 2012; Liu et al., 2012). Subsequent studies have found that the tau transgene is weakly expressed in other brain regions, which could influence the pattern of lesion progression (Yetman et al., 2016). However, when considered in light of the orderly neuroanatomic localization of tau lesions in interconnected brain regions in AD (Saper et al., 1987; Arnold et al., 1991; Braak and Braak, 1995), the experiments in mouse models implicate neuronal transport and cytotic mechanisms in the propagation of tau seeds within the nervous system. This possibility is indirectly supported by evidence for the neuronal trafficking of Aβ seeds, PrP-prions and other proteopathic seeds, as described above. Furthermore, in vivo imaging studies of the regional accumulation of pathogenic proteins in relation to the connectedness of the affected areas implicate the connectome in the systematic spread of seeds in AD and other neurodegenerative disorders (Bero et al., 2011; Zhou et al., 2012; Iturria-Medina et al., 2014; Raj et al., 2015).
Prion-like seeding and AD pathology in humans.
Between 1958 and 1985, approximately 30,000 children received a series of injections of cadaver-derived human growth hormone (c-hGH), in most instances to correct a deficiency in growth (Will, 2003; Brown et al., 2012). To obtain sufficient hormone for treatment, human pituitary glands were collected at autopsy, pooled into large batches, homogenized, and the c-hGH chemically extracted for injection. The treatment successfully stimulated growth, but years after treatment had ceased, a small percentage of the c-hGH recipients developed Creutzfeldt-Jakob disease (Brown et al., 2012). Subsequent studies have confirmed that the growth hormone was contaminated with PrP-prions, which presumably originated from pituitaries inadvertently obtained from patients who had died with prion disease (Jucker and Walker, 2015). In 1985, cadaver-derived hGH was replaced by recombinant growth hormone, thereby effectively eliminating the possibility that the therapeutic agent would be contaminated by prions (Brown et al., 2012).
Hypothesizing that pituitaries collected from AD patients were likely to be included in the batches for hormone extraction, Jaunmuktane, Collinge and colleagues sought evidence of AD-like pathology in eight c-hGH recipients who had died of prion disease approximately 30 years after treatment (Jaunmuktane et al., 2015). The cases ranged from 36 to 51 years of age at death – well before the lesions of idiopathic AD usually are evident – and they lacked the major genetic risk factors that would have predisposed them to early-onset AD. Along with the neurodegenerative changes typical of CJD, four of the subjects had extensive Aβ deposition in the brain in the form of both Aβ plaques and CAA, and two others had sparse Aβ deposits. Such Alzheimer-like Aβ-pathology was not present in control patients of similar age who had died of other (non-c-hGH-related) prion diseases. In addition, the frequent presence of Aβ-CAA is reminiscent of the increased vascular Aβ deposition seen in APP23 transgenic mice following peripheral administration of Aβ seeds (Eisele et al., 2010; Eisele et al., 2014; Burwinkel et al., 2018).
Significant Aβ deposition also has been reported in the brains of patients who died of CJD years after receiving PrP prion-contaminated dura mater transplants (Frontzek et al., 2016; Hamaguchi et al., 2016) or in c-hGH recipients who died of causes other than CJD (Ritchie et al., 2017). The most parsimonious explanation for the presence of Aβ-proteopathy in the recipients of human-derived biologics is that some batches of growth hormone and dura mater were contaminated with Aβ seeds in tissues originating from AD (or incipient AD) donors. This possibility is supported by evidence that some pituitary glands from AD patients (Irwin et al., 2013) (Figure 4A) and also samples from implicated c-hGH (Duyckaerts et al., 2018) and dura mater (Kovacs et al., 2016a) contain Aβ.
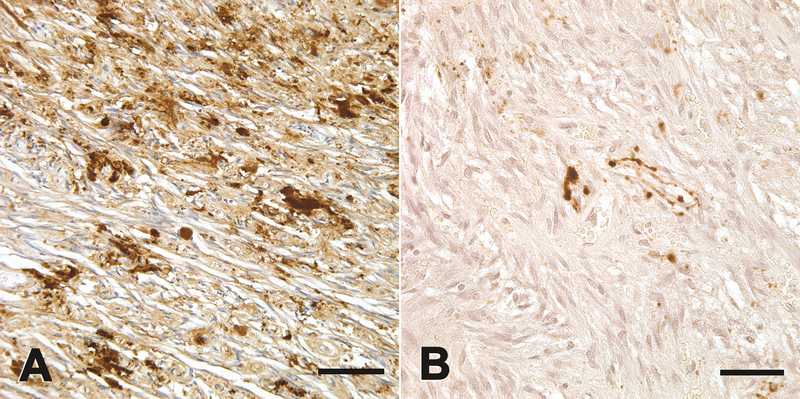
Figure 4.
Immunoreactive deposits (brown) of aggregated Aβ (A) and hyperphosphorylated tau (B) in the posterior lobe of the pituitary gland from a patient who had died with AD. Aβ was detected with antibody 82E1 to the N-terminal segment of Aβ, and tau was detected with antibody CP13 to an epitope around phosphoserine 202. The accumulation of Aβ and tau is generally mild in the pituitary. Hematoxylin counterstain (blue). Bars = 100μm in A and 50μm in B.
In light of experimental work on Aβ seeding in vivo (above), it is likely that a prion-like seeding mechanism underlies the development of Aβ-plaques and Aβ-CAA in recipients of c-hGH and dura mater transplants. Surprisingly, few of these iatrogenic CJD patients also had evidence of significant tauopathy (Jaunmuktane et al., 2015; Kovacs et al., 2016a; Duyckaerts et al., 2018). Mild tauopathy is present in AD-derived pituitaries (Hashizume et al., 2011; Irwin et al., 2013)(Figure 4B), and, as discussed above, tauopathy is directly seedable by aggregated tau in experimental models. Some c-hGH samples have been found to contain tau (Duyckaerts et al., 2018). Furthermore, experimental studies show that tau polymerization can be cross-seeded by aggregated Aβ (Vasconcelos et al., 2016) and that tauopathy is augmented by Aβ plaques in vivo (Pooler et al., 2015; Li et al., 2016).
Whether the Aβ-positive recipients of c-hGH or dura transplants would have manifested the full AD phenotype had they lived longer cannot be known. An analysis of pituitary hormone recipients in the US suggests that they are not more likely to develop AD than those in the general population (Irwin et al., 2013). However, longer term follow-up and investigation of c-hGH recipients in other countries, particularly where the processing of the hormone differed from that in the US (Brown et al., 2012), will be necessary to fully gauge the transmission risk of non-prion proteopathies in these instances.
Conclusions
The seeded propagation of misfolded Aβ is an early and obligatory occurrence in the cascade of events leading to the dementia of Alzheimer’s disease, but tauopathy is a critical downstream consequence that strongly impairs brain function. Both proteins have been shown to misfold, self-assemble and convey their abnormal properties to like proteins by a prion-like molecular mechanism. Therapeutic strategies for AD stemming from the prion paradigm include impeding the production or multimerization of the proteins, uncoupling the pathogenic link between abnormal Aβ and tau, and promoting the elimination of the seeds from the brain. Because Aβ-proteopathy and tauopathy each propagate by a prion-like mechanism of homologous protein corruption, it is likely that, once set in motion, the two pathologic processes advance more or less independently. If so, targeting Aβ should suffice for early prevention, but late-stage therapeutics will need to impede both branches of the cascade to be optimally effective. Another practical implication of the prion-like properties of misfolded Aβ and tau is to reinforce the importance of pristine instruments in neurosurgery. Finally, recognition of the prevalence of prionic mechanisms in neurodegenerative diseases could serve to integrate research efforts on these intractable disorders conceptually, experimentally and therapeutically.
Acknowledgments
I gratefully acknowledge insightful discussions with Mathias Jucker and the members of his laboratory in Tuebingen, and with David Lynn, Yury Chernoff, Anil Mehta and Harry LeVine. This work was supported in part by NIH grants P50 AG025688, RR00165, OD11132, and the Alexander von Humboldt Foundation.
References
- Agopian A & Guo Z (2012). Structural origin of polymorphism of Alzheimer’s amyloid beta-fibrils. Biochem J 447: 43–50. [DOI] [PubMed] [Google Scholar]
- Ahmed Z, Cooper J, Murray TK, et al. (2014). A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol 127: 667–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Flory J, et al. (1991). The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex 1: 103–116. [DOI] [PubMed] [Google Scholar]
- Attems J & Jellinger KA (2014). The overlap between vascular disease and Alzheimer’s disease--lessons from pathology. BMC Med 12: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audouard E, Houben S, Masaracchia C, et al. (2016). High-Molecular-Weight Paired Helical Filaments from Alzheimer Brain Induces Seeding of Wild-Type Mouse Tau into an Argyrophilic 4R Tau Pathology in Vivo. Am J Pathol 186: 2709–2722. [DOI] [PubMed] [Google Scholar]
- Baker HF, Ridley RM, Duchen LW, et al. (1994). Induction of beta (A4)-amyloid in primates by injection of Alzheimer’s disease brain homogenate. Comparison with transmission of spongiform encephalopathy. Mol Neurobiol 8: 25–39. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, et al. (2012). Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 367: 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benilova I, Gallardo R, Ungureanu AA, et al. (2014). The Alzheimer disease protective mutation A2T modulates kinetic and thermodynamic properties of amyloid-beta (Abeta) aggregation. J Biol Chem 289: 30977–30989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bero AW, Yan P, Roh JH, et al. (2011). Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci 14: 750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer LM, Hof PR, Purohit DP, et al. (1995). Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer’s disease. Arch Neurol 52: 81–88. [DOI] [PubMed] [Google Scholar]
- Biffi A & Greenberg SM (2011). Cerebral amyloid angiopathy: a systematic review. J Clin Neurol 7: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boluda S, Iba M, Zhang B, et al. (2015). Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer’s disease or corticobasal degeneration brains. Acta Neuropathol 129: 221–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H & Braak E (1995). Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 16: 271–278; discussion 278–284. [DOI] [PubMed] [Google Scholar]
- Brown P, Brandel JP, Sato T, et al. (2012). Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg Infect Dis 18: 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwinkel M, Lutzenberger M, Heppner FL, et al. (2018). Intravenous injection of beta-amyloid seeds promotes cerebral amyloid angiopathy (CAA). Acta Neuropathol Commun 6: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyukmihci N, Goehring-Harmon F & Marsh RF (1983). Neural pathogenesis of experimental scrapie after intraocular inoculation of hamsters. Exp Neurol 81: 396–406. [DOI] [PubMed] [Google Scholar]
- Charidimou A, Gang Q & Werring DJ (2012). Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry 83: 124–137. [DOI] [PubMed] [Google Scholar]
- Chouraki V & Seshadri S (2014). Genetics of Alzheimer’s disease. Adv Genet 87: 245–294. [DOI] [PubMed] [Google Scholar]
- Cintron AF, Dalal NV, Dooyema J, et al. (2015). Transport of cargo from periphery to brain by circulating monocytes. Brain Res 1622: 328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Akatsu H, Fraser G, et al. (2013). Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci U S A 110: 9535–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, et al. (2009). Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol 11: 909–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Hench J, Goedert M, et al. (2015). Invited review: Prion-like transmission and spreading of tau pathology. Neuropathol Appl Neurobiol 41: 47–58. [DOI] [PubMed] [Google Scholar]
- Clavaguera F, Hench J, Lavenir I, et al. (2014). Peripheral administration of tau aggregates triggers intracerebral tauopathy in transgenic mice. Acta Neuropathol 127: 299–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ML, Kim C, Haldiman T, et al. (2015). Rapidly progressive Alzheimer’s disease features distinct structures of amyloid-beta. Brain 138: 1009–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condello C, Lemmin T, Stohr J, et al. (2018). Structural heterogeneity and intersubject variability of Abeta in familial and sporadic Alzheimer’s disease. Proc Natl Acad Sci U S A 115: E782–E791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crary JF, Trojanowski JQ, Schneider JA, et al. (2014). Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128: 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther RA (1991). Straight and paired helical filaments in Alzheimer disease have a common structural unit. Proc Natl Acad Sci U S A 88: 2288–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal H, Dickson D, Fuld P, et al. (1988). Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology 38: 1682–1687. [DOI] [PubMed] [Google Scholar]
- Dartigues JF (2009). Alzheimer’s disease: a global challenge for the 21st century. Lancet Neurol 8: 1082–1083. [DOI] [PubMed] [Google Scholar]
- de Calignon A, Polydoro M, Suarez-Calvet M, et al. (2012). Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73: 685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeArmond SJ, Ironside JW, Bouzamondo-Bernstein E, et al. (2004). Neuropathology of Prion Diseases In: Prusiner SB (ed.) Prion Biology and Diseases. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- DeArmond SJ & Prusiner SB (1995). Etiology and pathogenesis of prion diseases. Am J Pathol 146: 785–811. [PMC free article] [PubMed] [Google Scholar]
- Deleault NR, Piro JR, Walsh DJ, et al. (2012). Isolation of phosphatidylethanolamine as a solitary cofactor for prion formation in the absence of nucleic acids. Proc Natl Acad Sci U S A 109: 8546–8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVos SL, Miller RL, Schoch KM, et al. (2017). Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci Transl Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fede G, Catania M, Morbin M, et al. (2009). A recessive mutation in the APP gene with dominant-negative effect on amyloidogenesis. Science 323: 1473–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diack AB, Alibhai JD, Barron R, et al. (2016). Insights into Mechanisms of Chronic Neurodegeneration. Int J Mol Sci 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domert J, Rao SB, Agholme L, et al. (2014). Spreading of amyloid-beta peptides via neuritic cell-to-cell transfer is dependent on insufficient cellular clearance. Neurobiol Dis 65: 82–92. [DOI] [PubMed] [Google Scholar]
- Drachman DA (2014). The amyloid hypothesis, time to move on: Amyloid is the downstream result, not cause, of Alzheimer’s disease. Alzheimers Dement 10: 372–380. [DOI] [PubMed] [Google Scholar]
- Duran-Aniotz C, Morales R, Moreno-Gonzalez I, et al. (2014). Aggregate-depleted brain fails to induce Abeta deposition in a mouse model of Alzheimer’s disease. PLoS One 9: e89014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Aniotz C, Morales R, Moreno-Gonzalez I, et al. (2013). Brains from non-Alzheimer’s individuals containing amyloid deposits accelerate Abeta deposition in vivo. Acta Neuropathol Commun 1: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyckaerts C, Delatour B & Potier MC (2009). Classification and basic pathology of Alzheimer disease. Acta Neuropathol 118: 5–36. [DOI] [PubMed] [Google Scholar]
- Duyckaerts C, Sazdovitch V, Ando K, et al. (2018). Neuropathology of iatrogenic Creutzfeldt-Jakob disease and immunoassay of French cadaver-sourced growth hormone batches suggest possible transmission of tauopathy and long incubation periods for the transmission of Abeta pathology. Acta Neuropathol 135: 201–212. [DOI] [PubMed] [Google Scholar]
- Eisele YS & Duyckaerts C (2016). Propagation of Ass pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol 131: 5–25. [DOI] [PubMed] [Google Scholar]
- Eisele YS, Fritschi SK, Hamaguchi T, et al. (2014). Multiple factors contribute to the peripheral induction of cerebral beta-amyloidosis. J Neurosci 34: 10264–10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Obermuller U, Heilbronner G, et al. (2010). Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science 330: 980–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D & Jucker M (2012). The amyloid state of proteins in human diseases. Cell 148: 1188–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon B, Cavallini A, Angers R, et al. (2015). Conformation determines the seeding potencies of native and recombinant Tau aggregates. J Biol Chem 290: 1049–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falker C, Hartmann A, Guett I, et al. (2016). Exosomal cellular prion protein drives fibrillization of amyloid beta and counteracts amyloid beta-mediated neurotoxicity. J Neurochem 137: 88–100. [DOI] [PubMed] [Google Scholar]
- Farquhar J & Gajdusek DC (1981). Kuru: Early Letters and Field Notes from the Collection of D. Gajdusek Carleton, New York, Raven Press. [Google Scholar]
- Fevrier B, Vilette D, Archer F, et al. (2004). Cells release prions in association with exosomes. Proc Natl Acad Sci U S A 101: 9683–9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler DM, Koulov AV, Balch WE, et al. (2007). Functional amyloid--from bacteria to humans. Trends Biochem Sci 32: 217–224. [DOI] [PubMed] [Google Scholar]
- Fraser H (1982). Neuronal spread of scrapie agent and targeting of lesions within the retino-tectal pathway. Nature 295: 149–150. [DOI] [PubMed] [Google Scholar]
- Fritschi SK, Cintron A, Ye L, et al. (2014a). Abeta seeds resist inactivation by formaldehyde. Acta Neuropathol 128: 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschi SK, Langer F, Kaeser SA, et al. (2014b). Highly potent soluble amyloid-beta seeds in human Alzheimer brain but not cerebrospinal fluid. Brain 137: 2909–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontzek K, Lutz MI, Aguzzi A, et al. (2016). Amyloid-beta pathology and cerebral amyloid angiopathy are frequent in iatrogenic Creutzfeldt-Jakob disease after dural grafting. Swiss Med Wkly 146: w14287. [DOI] [PubMed] [Google Scholar]
- Frost B, Jacks RL & Diamond MI (2009). Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem 284: 12845–12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman JL, Vaquer-Alicea J, White CL 3rd, et al. (2017). Widespread tau seeding activity at early Braak stages. Acta Neuropathol 133: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek DC (1977). Unconventional viruses and the origin and disappearance of kuru. Science 197: 943–960. [DOI] [PubMed] [Google Scholar]
- Gajdusek DC, Gibbs CJ & Alpers M (1966). Experimental transmission of a Kuru-like syndrome to chimpanzees. Nature 209: 794–796. [DOI] [PubMed] [Google Scholar]
- Gajdusek DC, Gibbs CJ Jr., Asher DM, et al. (1968). Transmission of experimental kuru to the spider monkey (Ateles geoffreyi). Science 162: 693–694. [DOI] [PubMed] [Google Scholar]
- Gambetti P, Cali I, Notari S, et al. (2011). Molecular biology and pathology of prion strains in sporadic human prion diseases. Acta Neuropathol 121: 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz M, Reynolds CA, Fratiglioni L, et al. (2006). Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry 63: 168–174. [DOI] [PubMed] [Google Scholar]
- Gerson J, Castillo-Carranza DL, Sengupta U, et al. (2016). Tau Oligomers Derived from Traumatic Brain Injury Cause Cognitive Impairment and Accelerate Onset of Pathology in Htau Mice. J Neurotrauma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerson J & Kayed R (2016). Therapeutic Approaches Targeting Pathological Tau Aggregates. Curr Pharm Des 22: 4028–4039. [DOI] [PubMed] [Google Scholar]
- Giaccone G, Mangieri M, Capobianco R, et al. (2008). Tauopathy in human and experimental variant Creutzfeldt-Jakob disease. Neurobiol Aging 29: 1864–1873. [DOI] [PubMed] [Google Scholar]
- Giaccone G, Morbin M, Moda F, et al. (2010). Neuropathology of the recessive A673V APP mutation: Alzheimer disease with distinctive features. Acta Neuropathol 120: 803–812. [DOI] [PubMed] [Google Scholar]
- Gibbs CJ Jr., Gajdusek DC, Asher DM, et al. (1968). Creutzfeldt-Jakob disease (spongiform encephalopathy): transmission to the chimpanzee. Science 161: 388–389. [DOI] [PubMed] [Google Scholar]
- Goedert M (2015). NEURODEGENERATION. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Abeta, tau, and alpha-synuclein. Science 349: 1255555. [DOI] [PubMed] [Google Scholar]
- Goudsmit J, Morrow CH, Asher DM, et al. (1980). Evidence for and against the transmissibility of Alzheimer disease. Neurology 30: 945–950. [DOI] [PubMed] [Google Scholar]
- Greenwald J & Riek R (2010). Biology of amyloid: structure, function, and regulation. Structure 18: 1244–1260. [DOI] [PubMed] [Google Scholar]
- Guo BB, Bellingham SA & Hill AF (2016a). Stimulating the Release of Exosomes Increases the Intercellular Transfer of Prions. J Biol Chem 291: 5128–5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JL & Lee VM (2011). Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem 286: 15317–15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JL, Narasimhan S, Changolkar L, et al. (2016b). Unique pathological tau conformers from Alzheimer’s brains transmit tau pathology in nontransgenic mice. J Exp Med 213: 2635–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Kaether C, Thinakaran G, et al. (2012). Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med 2: a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C & Selkoe DJ (2007). Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol 8: 101–112. [DOI] [PubMed] [Google Scholar]
- Hamaguchi T, Eisele YS, Varvel NH, et al. (2012). The presence of Abeta seeds, and not age per se, is critical to the initiation of Abeta deposition in the brain. Acta Neuropathol 123: 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi T, Taniguchi Y, Sakai K, et al. (2016). Significant association of cadaveric dura mater grafting with subpial Abeta deposition and meningeal amyloid angiopathy. Acta Neuropathol. [DOI] [PubMed] [Google Scholar]
- Hardy J (2006). A hundred years of Alzheimer’s disease research. Neuron 52: 3–13. [DOI] [PubMed] [Google Scholar]
- Hardy J & Selkoe DJ (2002). The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297: 353–356. [DOI] [PubMed] [Google Scholar]
- Harper JD & Lansbury PT Jr. (1997). Models of amyloid seeding in Alzheimer’s disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem 66: 385–407. [DOI] [PubMed] [Google Scholar]
- Hashizume M, Takagi J, Kanehira T, et al. (2011). Histologic study of age-related change in the posterior pituitary gland focusing on abnormal deposition of tau protein. Pathol Int 61: 13–18. [DOI] [PubMed] [Google Scholar]
- Hauw JJ & Duyckaerts C (2001). Alzheimer’s disease In: Duckett S & De La Torre JC (eds.) Pathology of the Aging Human Nervous System. 2 ed. Oxford: Oxford University Press. [Google Scholar]
- Head E, Lott IT, Wilcock DM, et al. (2016). Aging in Down Syndrome and the Development of Alzheimer’s Disease Neuropathology. Curr Alzheimer Res 13: 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner G, Eisele YS, Langer F, et al. (2013). Seeded strain-like transmission of beta-amyloid morphotypes in APP transgenic mice. EMBO Rep 14: 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock EM & Polymenidou M (2016). Prion-like propagation as a pathogenic principle in frontotemporal dementia. J Neurochem 138 Suppl 1: 163–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes BB, DeVos SL, Kfoury N, et al. (2013). Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc Natl Acad Sci U S A 110: E3138–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes BB, Furman JL, Mahan TE, et al. (2014). Proteopathic tau seeding predicts tauopathy in vivo. Proc Natl Acad Sci U S A 111: E4376–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Morris JC & Goate AM (2011). Alzheimer’s disease: the challenge of the second century. Sci Transl Med 3: 77sr71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu NW, Nicoll AJ, Zhang D, et al. (2014). mGlu5 receptors and cellular prion protein mediate amyloid-beta-facilitated synaptic long-term depression in vivo. Nat Commun 5: 3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Crick SL, Bu G, et al. (2009). Amyloid seeds formed by cellular uptake, concentration, and aggregation of the amyloid-beta peptide. Proc Natl Acad Sci U S A 106: 20324–20329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Zhou B, Wernig M, et al. (2017). ApoE2, ApoE3, and ApoE4 Differentially Stimulate APP Transcription and Abeta Secretion. Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries C & Kohli MA (2014). Rare Variants and Transcriptomics in Alzheimer disease. Curr Genet Med Rep 2: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba M, Guo JL, McBride JD, et al. (2013). Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J Neurosci 33: 1024–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba M, McBride JD, Guo JL, et al. (2015). Tau pathology spread in PS19 tau transgenic mice following locus coeruleus (LC) injections of synthetic tau fibrils is determined by the LC’s afferent and efferent connections. Acta Neuropathol 130: 349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironside JW, Head MW, Bell JE, et al. (2000). Laboratory diagnosis of variant Creutzfeldt-Jakob disease. Histopathology 37: 1–9. [DOI] [PubMed] [Google Scholar]
- Irwin DJ, Abrams JY, Schonberger LB, et al. (2013). Evaluation of potential infectivity of Alzheimer and Parkinson disease proteins in recipients of cadaver-derived human growth hormone. JAMA Neurol 70: 462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturria-Medina Y, Sotero RC, Toussaint PJ, et al. (2014). Epidemic spreading model to characterize misfolded proteins propagation in aging and associated neurodegenerative disorders. PLoS Comput Biol 10: e1003956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr. & Holtzman DM (2013). Biomarker modeling of Alzheimer’s disease. Neuron 80: 1347–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Knopman DS, Jagust WJ, et al. (2010). Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9: 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SJ, Kerridge C, Cooper J, et al. (2016). Short Fibrils Constitute the Major Species of Seed-Competent Tau in the Brains of Mice Transgenic for Human P301S Tau. J Neurosci 36: 762–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaunmuktane Z, Mead S, Ellis M, et al. (2015). Evidence for human transmission of amyloid-beta pathology and cerebral amyloid angiopathy. Nature 525: 247–250. [DOI] [PubMed] [Google Scholar]
- Jonsson T, Atwal JK, Steinberg S, et al. (2012). A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488: 96–99. [DOI] [PubMed] [Google Scholar]
- Joshi P, Benussi L, Furlan R, et al. (2015). Extracellular vesicles in Alzheimer’s disease: friends or foes? Focus on abeta-vesicle interaction. Int J Mol Sci 16: 4800–4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M & Walker LC (2013). Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M & Walker LC (2015). Neurodegeneration: Amyloid-beta pathology induced in humans. Nature 525: 193–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser SA, Herzig MC, Coomaraswamy J, et al. (2007). Cystatin C modulates cerebral beta-amyloidosis. Nat Genet 39: 1437–1439. [DOI] [PubMed] [Google Scholar]
- Kalimo H, Lalowski M, Bogdanovic N, et al. (2013). The Arctic AbetaPP mutation leads to Alzheimer’s disease pathology with highly variable topographic deposition of differentially truncated Abeta. Acta Neuropathol Commun 1: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MD, Lipinski WJ, Callahan MJ, et al. (2000). Evidence for seeding of beta -amyloid by intracerebral infusion of Alzheimer brain extracts in beta -amyloid precursor protein-transgenic mice. J Neurosci 20: 3606–3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapasi A & Schneider JA (2016). Vascular contributions to cognitive impairment, clinical Alzheimer’s disease, and dementia in older persons. Biochim Biophys Acta 1862: 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman SK, Sanders DW, Thomas TL, et al. (2016). Tau Prion Strains Dictate Patterns of Cell Pathology, Progression Rate, and Regional Vulnerability In Vivo. Neuron 92: 796–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawas CH & Katzman R (1999). Epidemiology of dementia and Alzheimer disease In: Terry RD, Katzman R, Bick KL, et al. (eds.) Alzheimer Disease. Philadelphia: Lippincott Williams and Wilkins. [Google Scholar]
- Killin LO, Starr JM, Shiue IJ, et al. (2016). Environmental risk factors for dementia: a systematic review. BMC Geriatr 16: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin RH & Walker CA (1986). Pathogenesis of scrapie (strain 263K) in hamsters infected intracerebrally, intraperitoneally or intraocularly. J Gen Virol 67 ( Pt 2): 255–263. [DOI] [PubMed] [Google Scholar]
- Kodali R, Williams AD, Chemuru S, et al. (2010). Abeta(1–40) forms five distinct amyloid structures whose beta-sheet contents and fibril stabilities are correlated. J Mol Biol 401: 503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG (2015). Invited review: Neuropathology of tauopathies: principles and practice. Neuropathol Appl Neurobiol 41: 3–23. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Lutz MI, Ricken G, et al. (2016a). Dura mater is a potential source of Abeta seeds. Acta Neuropathol 131: 911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, Rahimi J, Strobel T, et al. (2016b). Tau Pathology in Creutzfeldt-Jakob Disease Revisited. Brain Pathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafortune L, Martin S, Kelly S, et al. (2016). Behavioural Risk Factors in Mid-Life Associated with Successful Ageing, Disability, Dementia and Frailty in Later Life: A Rapid Systematic Review. PLoS One 11: e0144405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, et al. (1998). Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A 95: 6448–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer F, Eisele YS, Fritschi SK, et al. (2011). Soluble Abeta seeds are potent inducers of cerebral beta-amyloid deposition. J Neurosci 31: 14488–14495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, et al. (2012). Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci Rep 2: 700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren J (2014). Cellular prion protein as a therapeutic target in Alzheimer’s disease. J Alzheimers Dis 38: 227–244. [DOI] [PubMed] [Google Scholar]
- Lee VM-Y, Goedert M & Trojanowski JQ (2001). Neurodegenerative Tauopathies. Annual Review of Neuroscience 24: 1121–1159. [DOI] [PubMed] [Google Scholar]
- Legname G, Baskakov IV, Nguyen HO, et al. (2004). Synthetic mammalian prions. Science 305: 673–676. [DOI] [PubMed] [Google Scholar]
- Lewczuk P, Mroczko B, Fagan A, et al. (2015). Biomarkers of Alzheimer’s disease and mild cognitive impairment: a current perspective. Adv Med Sci 60: 76–82. [DOI] [PubMed] [Google Scholar]
- Li T, Braunstein KE, Zhang J, et al. (2016). The neuritic plaque facilitates pathological conversion of tau in an Alzheimer’s disease mouse model. Nat Commun 7: 12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberski PP, Hainfellner JA, Sikorska B, et al. (2012). Prion protein (PrP) deposits in the tectum of experimental Gerstmann-Straussler-Scheinker disease following intraocular inoculation. Folia Neuropathol 50: 85–88. [PubMed] [Google Scholar]
- Liu CC, Kanekiyo T, Xu H, et al. (2013). Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 9: 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Drouet V, Wu JW, et al. (2012). Trans-synaptic spread of tau pathology in vivo. PLoS One 7: e31302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JX, Qiang W, Yau WM, et al. (2013). Molecular structure of beta-amyloid fibrils in Alzheimer’s disease brain tissue. Cell 154: 1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundmark K, Westermark GT, Nystrom S, et al. (2002). Transmissibility of systemic amyloidosis by a prion-like mechanism. Proc Natl Acad Sci U S A 99: 6979–6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DM & Yates PO (1986). Neurotransmitter deficits in Alzheimer’s disease and in other dementing disorders. Hum Neurobiol 5: 147–158. [PubMed] [Google Scholar]
- Marzesco AM, Flotenmeyer M, Buhler A, et al. (2016). Highly potent intracellular membrane-associated Abeta seeds. Sci Rep 6: 28125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matveev SV, Spielmann HP, Metts BM, et al. (2014). A distinct subfraction of Abeta is responsible for the high-affinity Pittsburgh compound B-binding site in Alzheimer’s disease brain. J Neurochem 131: 356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKintosh E, Tabrizi SJ & Collinge J (2003). Prion diseases. J Neurovirol 9: 183–193. [DOI] [PubMed] [Google Scholar]
- Meinhardt J, Sachse C, Hortschansky P, et al. (2009). Abeta(1–40) fibril polymorphism implies diverse interaction patterns in amyloid fibrils. J Mol Biol 386: 869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Coomaraswamy J, Bolmont T, et al. (2006). Exogenous induction of cerebral {beta}-amyloidogenesis is governed by agent and host. Science 313: 1781–1784. [DOI] [PubMed] [Google Scholar]
- Miller Y, Ma B & Nussinov R (2010). Polymorphism in Alzheimer Abeta amyloid organization reflects conformational selection in a rugged energy landscape. Chem Rev 110: 4820–4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirbaha H, Holmes BB, Sanders DW, et al. (2015). Tau Trimers Are the Minimal Propagation Unit Spontaneously Internalized to Seed Intracellular Aggregation. J Biol Chem 290: 14893–14903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales R, Bravo-Alegria J, Duran-Aniotz C, et al. (2015a). Titration of biologically active amyloid-beta seeds in a transgenic mouse model of Alzheimer’s disease. Sci Rep 5: 9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales R, Callegari K & Soto C (2015b). Prion-like features of misfolded Abeta and tau aggregates. Virus Res 207: 106–112. [DOI] [PubMed] [Google Scholar]
- Morales R, Duran-Aniotz C, Castilla J, et al. (2012). De novo induction of amyloid-beta deposition in vivo. Mol Psychiatry 17: 1347–1353. [DOI] [PubMed] [Google Scholar]
- Morgado I & Garvey M (2015). Lipids in Amyloid-beta Processing, Aggregation, and Toxicity. Adv Exp Med Biol 855: 67–94. [DOI] [PubMed] [Google Scholar]
- Narasimhan S, Guo JL, Changolkar L, et al. (2017). Pathological Tau Strains from Human Brains Recapitulate the Diversity of Tauopathies in Nontransgenic Mouse Brain. J Neurosci 37: 11406–11423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath S, Agholme L, Kurudenkandy FR, et al. (2012). Spreading of neurodegenerative pathology via neuron-to-neuron transmission of beta-amyloid. J Neurosci 32: 8767–8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, et al. (2012). Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 71: 362–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieznanski K, Choi JK, Chen S, et al. (2012). Soluble prion protein inhibits amyloid-beta (Abeta) fibrillization and toxicity. J Biol Chem 287: 33104–33108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson KP, Aslund A, Berg I, et al. (2007). Imaging distinct conformational states of amyloid-beta fibrils in Alzheimer’s disease using novel luminescent probes. ACS Chem Biol 2: 553–560. [DOI] [PubMed] [Google Scholar]
- Novotny R, Langer F, Mahler J, et al. (2016). Conversion of Synthetic Abeta to In Vivo Active Seeds and Amyloid Plaque Formation in a Hippocampal Slice Culture Model. J Neurosci 36: 5084–5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson B, Lautner R, Andreasson U, et al. (2016). CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol 15: 673–684. [DOI] [PubMed] [Google Scholar]
- Paravastu AK, Leapman RD, Yau WM, et al. (2008). Molecular structural basis for polymorphism in Alzheimer’s beta-amyloid fibrils. Proc Natl Acad Sci U S A 105: 18349–18354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeraer E, Bottelbergs A, Van Kolen K, et al. (2015). Intracerebral injection of preformed synthetic tau fibrils initiates widespread tauopathy and neuronal loss in the brains of tau transgenic mice. Neurobiol Dis 73: 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz D, Williamson RA, Legname G, et al. (2002). A change in the conformation of prions accompanies the emergence of a new prion strain. Neuron 34: 921–932. [DOI] [PubMed] [Google Scholar]
- Petkova AT, Leapman RD, Guo Z, et al. (2005). Self-propagating, molecular-level polymorphism in Alzheimer’s beta-amyloid fibrils. Science 307: 262–265. [DOI] [PubMed] [Google Scholar]
- Piccardo P, King D, Telling G, et al. (2013). Dissociation of prion protein amyloid seeding from transmission of a spongiform encephalopathy. J Virol 87: 12349–12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccini A, Russo C, Gliozzi A, et al. (2005). Beta-Amyloid is different in normal aging and in Alzheimer disease. J Biol Chem 34: 186–192. [DOI] [PubMed] [Google Scholar]
- Pike CJ (2017). Sex and the development of Alzheimer’s disease. J Neurosci Res 95: 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanco JC, Scicluna BJ, Hill AF, et al. (2016). Extracellular Vesicles Isolated from the Brains of rTg4510 Mice Seed Tau Protein Aggregation in a Threshold-dependent Manner. J Biol Chem 291: 12445–12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooler AM, Polydoro M, Maury EA, et al. (2015). Amyloid accelerates tau propagation and toxicity in a model of early Alzheimer’s disease. Acta Neuropathol Commun 3: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portelius E, Lashley T, Westerlund A, et al. (2015). Brain amyloid-beta fragment signatures in pathological ageing and Alzheimer’s disease by hybrid immunoprecipitation mass spectrometry. Neurodegener Dis 15: 50–57. [DOI] [PubMed] [Google Scholar]
- Potter H & Wisniewski T (2012). Apolipoprotein e: essential catalyst of the Alzheimer amyloid cascade. Int J Alzheimers Dis 2012: 489428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Properzi F, Logozzi M, Abdel-Haq H, et al. (2015). Detection of exosomal prions in blood by immunochemistry techniques. J Gen Virol 96: 1969–1974. [DOI] [PubMed] [Google Scholar]
- Prusiner SB (1984). Some speculations about prions, amyloid, and Alzheimer’s disease. N Engl J Med 310: 661–663. [DOI] [PubMed] [Google Scholar]
- Prusiner SB (2013). Biology and genetics of prions causing neurodegeneration. Annu Rev Genet 47: 601–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang W, Yau WM, Lu JX, et al. (2017). Structural variation in amyloid-beta fibrils from Alzheimer’s disease clinical subtypes. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, LoCastro E, Kuceyeski A, et al. (2015). Network Diffusion Model of Progression Predicts Longitudinal Patterns of Atrophy and Metabolism in Alzheimer’s Disease. Cell Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran L, Honsho M, Zahn TR, et al. (2006). Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A 103: 11172–11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, Race B, Phillips K, et al. (2014). Distinct patterns of spread of prion infection in brains of mice expressing anchorless or anchored forms of prion protein. Acta Neuropathol Commun 2: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen J, Mahler J, Beschorner N, et al. (2017). Amyloid polymorphisms constitute distinct clouds of conformational variants in different etiological subtypes of Alzheimer’s disease. Proc Natl Acad Sci U S A 114: 13018–13023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, Brayne C & Mayeux R (2011). Epidemiology of Alzheimer disease. Nat Rev Neurol 7: 137–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Bilgel M, Moghekar A, et al. (2015). Changes in Abeta biomarkers and associations with APOE genotype in 2 longitudinal cohorts. Neurobiol Aging 36: 2333–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revesz T, Ghiso J, Lashley T, et al. (2003). Cerebral amyloid angiopathies: a pathologic, biochemical, and genetic view. J Neuropathol Exp Neurol 62: 885–898. [DOI] [PubMed] [Google Scholar]
- Rey NL, Steiner JA, Maroof N, et al. (2016). Widespread transneuronal propagation of alpha-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease. J Exp Med 213: 1759–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie DL, Adlard P, Peden AH, et al. (2017). Amyloid-beta accumulation in the CNS in human growth hormone recipients in the UK. Acta Neuropathol 134: 221–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnback A, Sagelius H, Bergstedt KD, et al. (2012). Amyloid neuropathology in the single Arctic APP transgenic model affects interconnected brain regions. Neurobiol Aging 33: 831 e811–839. [DOI] [PubMed] [Google Scholar]
- Rosen RF, Ciliax BJ, Wingo TS, et al. (2010). Deficient high-affinity binding of Pittsburgh compound B in a case of Alzheimer’s disease. Acta Neuropathol 119: 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RF, Fritz JJ, Dooyema J, et al. (2012). Exogenous seeding of cerebral beta-amyloid deposition in betaAPP-transgenic rats. J Neurochem 120: 660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RF, Tomidokoro Y, Farberg AS, et al. (2016). Comparative pathobiology of beta-amyloid and the unique susceptibility of humans to Alzheimer’s disease. Neurobiol Aging 44: 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RF, Walker LC & Levine H 3rd (2011). PIB binding in aged primate brain: enrichment of high-affinity sites in humans with Alzheimer’s disease. Neurobiol Aging 32: 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth JV, Griffiths HH, Watt NT, et al. (2013). Prion protein-mediated toxicity of amyloid-beta oligomers requires lipid rafts and the transmembrane LRP1. J Biol Chem 288: 8935–8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar SV & Strittmatter SM (2016). Cellular prion protein as a receptor for amyloid-beta oligomers in Alzheimer’s disease. Biochem Biophys Res Commun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders DW, Kaufman SK, DeVos SL, et al. (2014). Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron 82: 1271–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Wainer BH & German DC (1987). Axonal and transneuronal transport in the transmission of neurological disease: potential role in system degenerations, including Alzheimer’s disease. Neuroscience 23: 389–398. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ (2011). Resolving controversies on the path to Alzheimer’s therapeutics. Nat Med 17: 1060–1065. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ & Hardy J (2016). The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8: 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherzai A, Edland SD, Masliah E, et al. (2013). Spongiform change in dementia with Lewy bodies and Alzheimer disease. Alzheimer Dis Assoc Disord 27: 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira JR, Raymond GJ, Hughson AG, et al. (2005). The most infectious prion protein particles. Nature 437: 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe JD, Benson MD, Buxbaum JN, et al. (2016). Amyloid fibril proteins and amyloidosis: chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid 23: 209–213. [DOI] [PubMed] [Google Scholar]
- Smith TW, Anwer U, DeGirolami U, et al. (1987). Vacuolar change in Alzheimer’s disease. Arch Neurol 44: 1225–1228. [DOI] [PubMed] [Google Scholar]
- Spillantini MG & Goedert M (2013). Tau pathology and neurodegeneration. Lancet Neurol 12: 609–622. [DOI] [PubMed] [Google Scholar]
- Spirig T, Ovchinnikova O, Vagt T, et al. (2014). Direct evidence for self-propagation of different amyloid-beta fibril conformations. Neurodegener Dis 14: 151–159. [DOI] [PubMed] [Google Scholar]
- Stancu IC, Vasconcelos B, Ris L, et al. (2015). Templated misfolding of Tau by prion-like seeding along neuronal connections impairs neuronal network function and associated behavioral outcomes in Tau transgenic mice. Acta Neuropathol 129: 875–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohr J, Condello C, Watts JC, et al. (2014). Distinct synthetic Abeta prion strains producing different amyloid deposits in bigenic mice. Proc Natl Acad Sci U S A 111: 10329–10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohr J, Watts JC, Mensinger ZL, et al. (2012). Purified and synthetic Alzheimer’s amyloid beta (Abeta) prions. Proc Natl Acad Sci U S A 109: 11025–11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Commins C, DeVos SL, et al. (2016). Seed-competent HMW tau species accumulates in the cerebrospinal fluid of Alzheimer’s disease mouse model and human patients. Ann Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Collins SR, Toyama BH, et al. (2006). The physical basis of how prion conformations determine strain phenotypes. Nature 442: 585–589. [DOI] [PubMed] [Google Scholar]
- Tanzi RE (2012). The genetics of Alzheimer disease. Cold Spring Harb Perspect Med 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayeb-Fligelman E, Tabachnikov O, Moshe A, et al. (2017). The cytotoxic Staphylococcus aureus PSMalpha3 reveals a cross-alpha amyloid-like fibril. Science 355: 831–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD, Masliah E & Hansen LA (1999). The neuropathology of Alzheimer disease and the structural basis of cognitive alterations In: Terry RD, Katzman R, Bick KL, et al. (eds.) Alzheimer Disease. 2 ed. Philadelphia: Lippincott Williams and Wilkins. [Google Scholar]
- Thal DR, Capetillo-Zarate E, Del Tredici K, et al. (2006). The development of amyloid beta protein deposits in the aged brain. Sci Aging Knowledge Environ 2006: re1. [DOI] [PubMed] [Google Scholar]
- Tripathi M & Vibha D (2009). Reversible dementias. Indian J Psychiatry 51 Suppl 1: S52–55. [PMC free article] [PubMed] [Google Scholar]
- Tycko R (2015). Amyloid polymorphism: structural basis and neurobiological relevance. Neuron 86: 632–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tycko R (2016). Alzheimer’s disease: Structure of aggregates revealed. Nature 537: 492–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JW, Nygaard HB, Heiss JK, et al. (2012). Alzheimer amyloid-beta oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat Neurosci 15: 1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos B, Stancu IC, Buist A, et al. (2016). Heterotypic seeding of Tau fibrillization by pre-aggregated Abeta provides potent seeds for prion-like seeding and propagation of Tau-pathology in vivo. Acta Neuropathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Dore V, Bourgeat P, et al. (2017). Abeta-amyloid and Tau Imaging in Dementia. Semin Nucl Med 47: 75–88. [DOI] [PubMed] [Google Scholar]
- Vinters HV (2015). Emerging concepts in Alzheimer’s disease. Annu Rev Pathol 10: 291–319. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP & Hedley-White ET (2001). Dementia. Pathology of the Aging Human Nervous System. New York: Oxford University Press. [Google Scholar]
- Walker LC, Callahan MJ, Bian F, et al. (2002). Exogenous induction of cerebral beta-amyloidosis in betaAPP-transgenic mice. Peptides 23: 1241–1247. [DOI] [PubMed] [Google Scholar]
- Walker LC & Jucker M (2015). Neurodegenerative diseases: expanding the prion concept. Annu Rev Neurosci 38: 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC & Jucker M (2017). The Exceptional Vulnerability of Humans to Alzheimer’s Disease. Trends Mol Med 23: 534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC & LeVine H (2000). The cerebral proteopathies: neurodegenerative disorders of protein conformation and assembly. Mol Neurobiol 21: 83–95. [DOI] [PubMed] [Google Scholar]
- Walker LC, Levine H 3rd, Mattson MP, et al. (2006). Inducible proteopathies. Trends Neurosci 29: 438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, Pahnke J, Madauss M, et al. (2000). Apolipoprotein E4 promotes the early deposition of Abeta42 and then Abeta40 in the elderly. Acta Neuropathol 100: 36–42. [DOI] [PubMed] [Google Scholar]
- Walker LC, Schelle J & Jucker M (2016). The Prion-Like Properties of Amyloid-beta Assemblies: Implications for Alzheimer’s Disease. Cold Spring Harb Perspect Med 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Wang X, Yuan CG, et al. (2010). Generating a prion with bacterially expressed recombinant prion protein. Science 327: 1132–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Balaji V, Kaniyappan S, et al. (2017). The release and trans-synaptic transmission of Tau via exosomes. Mol Neurodegener 12: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warzok RW, Kessler C, Apel G, et al. (1998). Apolipoprotein E4 promotes incipient Alzheimer pathology in the elderly. Alzheimer Dis Assoc Disord 12: 33–39. [DOI] [PubMed] [Google Scholar]
- Watts JC, Condello C, Stohr J, et al. (2014). Serial propagation of distinct strains of Abeta prions from Alzheimer’s disease patients. Proc Natl Acad Sci U S A 111: 10323–10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JC, Giles K, Grillo SK, et al. (2011). Bioluminescence imaging of Abeta deposition in bigenic mouse models of Alzheimer’s disease. Proc Natl Acad Sci U S A 108: 2528–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MW, Veitch DP, Aisen PS, et al. (2013). The Alzheimer’s Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement 9: e111–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann C (2004). The state of the prion. Nat Rev Microbiol 2: 861–871. [DOI] [PubMed] [Google Scholar]
- Westermark GT, Fandrich M, Lundmark K, et al. (2017). Noncerebral Amyloidoses: Aspects on Seeding, Cross-Seeding, and Transmission. Cold Spring Harb Perspect Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock GK & Esiri MM (1982). Plaques, tangles and dementia. A quantitative study. J Neurol Sci 56: 343–356. [DOI] [PubMed] [Google Scholar]
- Will RG (2003). Acquired prion disease: iatrogenic CJD, variant CJD, kuru. Br Med Bull 66: 255–265. [DOI] [PubMed] [Google Scholar]
- Wimo A, Jonsson L, Bond J, et al. (2013). The worldwide economic impact of dementia 2010. Alzheimers Dement 9: 1–11 e13. [DOI] [PubMed] [Google Scholar]
- Wingo TS, Lah JJ, Levey AI, et al. (2012). Autosomal recessive causes likely in early-onset Alzheimer disease. Arch Neurol 69: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman FK, Cancellotti E, Piccardo P, et al. (2015). The glycosylation status of PrPC is a key factor in determining transmissible spongiform encephalopathy transmission between species. J Virol 89: 4738–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski HM, Bancher C, Barcikowska M, et al. (1989). Spectrum of morphological appearance of amyloid deposits in Alzheimer’s disease. Acta Neuropathol 78: 337–347. [DOI] [PubMed] [Google Scholar]
- Woerman AL, Aoyagi A, Patel S, et al. (2016). Tau prions from Alzheimer’s disease and chronic traumatic encephalopathy patients propagate in cultured cells. Proc Natl Acad Sci U S A 113: E8187–E8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JW, Herman M, Liu L, et al. (2013). Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J Biol Chem 288: 1856–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JW, Hussaini SA, Bastille IM, et al. (2016). Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H, Ban T, Morigaki K, et al. (2007). Visualization and Classification of Amyloid beta Supramolecular Assemblies. Biochemistry. [DOI] [PubMed] [Google Scholar]
- Yang T, Li S, Xu H, et al. (2017). Large Soluble Oligomers of Amyloid beta-Protein from Alzheimer Brain Are Far Less Neuroactive Than the Smaller Oligomers to Which They Dissociate. J Neurosci 37: 152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Fritschi SK, Schelle J, et al. (2015a). Persistence of Abeta seeds in APP null mouse brain. Nat Neurosci 18: 1559–1561. [DOI] [PubMed] [Google Scholar]
- Ye L, Hamaguchi T, Fritschi SK, et al. (2015b). Progression of Seed-Induced Abeta Deposition within the Limbic Connectome. Brain Pathol 25: 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetman MJ, Lillehaug S, Bjaalie JG, et al. (2016). Transgene expression in the Nop-tTA driver line is not inherently restricted to the entorhinal cortex. Brain Struct Funct 221: 2231–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JT, Tan L & Hardy J (2014). Apolipoprotein E in Alzheimer’s disease: an update. Annu Rev Neurosci 37: 79–100. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang F, Wang X, et al. (2014). Comparison of 2 synthetically generated recombinant prions. Prion 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Gennatas ED, Kramer JH, et al. (2012). Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron 73: 1216–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]