Abstract
Context
Epidemiologic data link psychological stress to adiposity. The underlying mechanisms remain uncertain.
Objectives
To test whether (i) higher activity of the amygdala, a neural center involved in the response to stress, associates with greater visceral adipose tissue (VAT) volumes and (ii) this association is mediated by increased bone marrow activity.
Setting
Massachusetts General Hospital, Boston, Massachusetts.
Patients
Two hundred forty-six patients without active oncologic, cardiovascular, or inflammatory disease who underwent clinical 18F-fluorodeoxyglucose positron emission tomography/computed tomography imaging were studied. VAT imaging was repeated ∼1 year later in 68 subjects.
Design
Metabolic activity of the amygdala (AmygA), hematopoietic tissue activity, and adiposity volumes were measured with validated methods.
Main Outcome Measure
The relationship between AmygA and baseline and follow-up VAT.
Results
AmygA associated with baseline body mass index (standardized β = 0.15; P = 0.01), VAT (0.19; P = 0.002), and VAT/subcutaneous adipose tissue ratio (0.20; P = 0.002), all remaining significant after adjustment for age and sex. AmygA also associated with bone marrow activity (0.15; P = 0.01), which in turn associated with VAT (0.34; P < 0.001). Furthermore, path analysis showed that 48% of the relationship between AmygA and baseline VAT was mediated by increased bone marrow activity (P = 0.007). Moreover, AmygA associated with achieved VAT after 1 year (P = 0.02) after adjusting for age, sex, and baseline VAT.
Conclusions
These results suggest a neurobiological pathway involving the amygdala and bone marrow linking psychosocial stress to adiposity in humans. Future studies should test whether targeting this mechanism attenuates adiposity and its complications.
We demonstrated that higher stress-associated neural activity was associated with visceral adiposity through bone marrow activity, suggesting a role for stress-related inflammation in central obesity.
Obesity is a rapidly growing threat to health across the world (1, 2), which independently increases the risk for cardiovascular disease (CVD), malignancy, and all-cause mortality (3, 4). Body fat distribution, specifically increased visceral adipose tissue (VAT), is a better predictor of disease than body mass index (BMI) or total body fat mass (5–8). A large body of evidence demonstrates that in addition to adverse health behaviors (e.g., poor diet and physical inactivity), chronic psychosocial stress is an important independent risk factor for adiposity (9, 10). Nevertheless, the assessment and management of stress are not core components of most current weight loss approaches, partly because of inadequate understanding of the mechanisms connecting stress to adiposity. Clarification of these mechanisms may facilitate lifestyle and pharmacologic interventions to limit the expansion of adiposity and its complications.
It is a common misconception that stress leads to increased adiposity solely through an increase in adverse health behaviors, particularly increased intake of less healthy foods. In fact, animals chronically exposed to stressful stimuli have increased visceral fat depots despite comparable or lower food intake (11, 12). Similarly, in some but not all human studies, stress associates with adiposity and metabolic impairment independently of diet and physical activity, and it lowers the threshold at which adverse adiposity develops for a given diet (13–15). Release of monocytes from hematopoietic tissues appears to play an important role in this connection. In animal models, stress triggers increased hematopoietic stem and progenitor cell proliferation within the bone marrow and accelerates immune cellular output (16–20). These stress-induced monocytes subsequently infiltrate VAT, prompt adipocyte dysfunction, and promote VAT expansion (21, 22). However, it is unknown whether a homologous association among stress, hematopoietic activity, and VAT exists in humans. Moreover, the role of the brain in this mechanism remains undetermined.
Studies evaluating the effect of stress on human physiology have been facilitated by advanced neuroimaging methods, which provide objective measures of the neurobiological response to stress (23–26). The amygdala is part of an endogenous circuitry within the brain that mediates neuroendocrine, autonomic, and behavioral changes in response to stress (27, 28). Resting metabolic activity in the amygdala (AmygA) can be reproducibly quantified using 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) imaging. AmygA is relatively stable over time (29, 30) and associates with an individual’s perceived stress (25). The amygdala is conditioned by chronic stress, and increased amygdalar metabolic activity and activation have been noted in chronic stress-related disorders such as posttraumatic stress disorder and anxiety (23, 31–33). Furthermore, AmygA is linked to adverse pathologic consequences of chronic stress (25, 26). Our group recently observed that higher AmygA leads to future CVD events in humans through a serial mechanism that involves increased hematopoietic tissue activity, with the putative release of inflammatory white blood cells, and increased arterial inflammation and predicts the development of incident diabetes mellitus independently of adiposity (25, 26). Moreover, using positron emission tomography (PET)/CT, AmygA and bone marrow activity can be quantified by 18F-FDG uptake, along with simultaneous measurement of adipose tissue volumes with CT, thereby making this modality uniquely suitable for investigating the possible biological connections among these systems. Accordingly, we analyzed the data from brain and body 18F-FDG PET/CT imaging to evaluate the hypotheses that (i) higher AmygA associates with greater baseline VAT volume and with greater achieved VAT volume after 1 year and (ii) increased bone marrow activity mediates the relationship between AmygA and VAT.
Methods
Study cohort
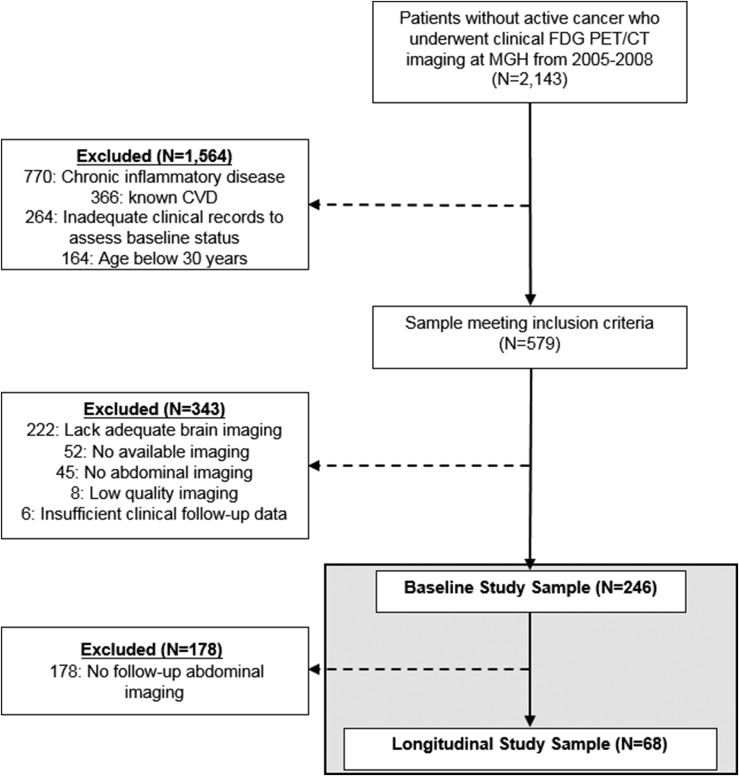
The study cohort (N = 246) was identified from a database of 2143 patients without active oncologic disease who underwent clinical 18F-FDG PET/CT imaging at Massachusetts General Hospital from 2005 to 2008 (Fig. 1), predominantly for cancer screening or malignancy surveillance. In addition, predefined inclusion criteria were (i) age >30 years, (ii) absence of prior CVD, (iii) absence of acute or chronic inflammatory or autoimmune disease, (iv) absence of prior malignancy or remission for ≥1 year before imaging and throughout the follow-up period, and (v) availability of adequate brain and adipose tissue images. Furthermore, a subset of 68 subjects underwent clinically indicated follow-up imaging 343 ± 105 days (mean ± SD) after baseline imaging. The Partners Human Research Committee approved the protocol for this retrospective study.
Figure 1.
Subject selection schema. MGH, Massachusetts General Hospital.
18F-FDG PET/CT imaging protocol
Whole-body 18F-FDG PET/CT imaging was performed using a standard integrated scanner (Biograph 64; Siemens Healthcare, Erlangen, Germany; or similar). 18F-FDG (∼370 MBq) was administered intravenously after an overnight fast. PET images were acquired approximately 60 minutes later in three-dimensional mode. A low-dose, nongated, noncontrast CT (120 keV, ∼50 mA) was performed for attenuation correction prior to PET imaging.
Measurement of adipose tissue volumes
Adipose tissue volumes were measured on CT by investigators (B.T., Y.W.) who were blinded to clinical data and PET imaging measurements. Abdominal VAT and subcutaneous adipose tissue (SAT) were measured using previously validated methods on a dedicated offline workstation (Siemens Medical Solutions, Forchheim, Germany) on a single slice at the level of the umbilicus (34). Adipose tissue was identified using a threshold between −195 and −45 Hounsfield units. To separate visceral from subcutaneous fat, the abdominal muscular wall separating the two compartments was manually traced. VAT and SAT were calculated in cubic centimeters, and total fat was calculated as the sum of both depots. Lean tissue volume was calculated by quantifying the entire volume of tissue in the slice and subtracting the total adipose volume.
Measurement of brain metabolic activity
18F-FDG PET imaging analysis was performed by a radiologist (A.I.) who was blinded to the clinical data and adipose volume measurements, using previously validated methods (25). 18F-FDG uptake in the amygdala was evaluated by placing circular regions of interest with an approximately 15-mm radius over the right and left amygdalae and measuring the mean tracer accumulation, as quantified by standardized uptake value (SUV). AmygA was defined as the average of the mean SUV from bilateral amygdalae corrected for background cerebral activity (mean temporal lobe SUV) (35).
Measurement of hematopoietic tissue activity
Bone marrow activity was evaluated using previously validated methods (A.I.) (18, 25). SUVs from the target tissue (bone marrow) were derived, and the target/background ratio was calculated by correcting for venous blood background activity.
Statistical analyses
Statistical analyses were performed using SPSS (version 23; IBM Corp, Armonk, NY). Continuous variables are given as mean and SD or, when not normally distributed, as median and interquartile range (25th, 75th percentiles). Linear regression was used to test for associations, measured as standardized β with 95% CIs. All multivariable analyses incorporated age and sex as covariates; baseline adiposity measures and prior 18F-FDG PET/CT images were also used as covariates when appropriate.
Mediation (path) analysis was performed using the SPSS PROCESS macro (Preacher and Hayes), which employs an ordinary least squares or logistic regression‒based path framework to estimate direct and indirect effects and produces CIs from 5000 bias-corrected bootstrap samples. We tested a hypothesized single mediator path: amygdalar activity → bone marrow activity → VAT. Age and sex were entered as covariables in mediation models. Statistical significance was determined as a two-sided P value <0.05 for all analyses.
Results
Baseline characteristics of study cohort
A total of 246 individuals, 40.7% male, were included. Median age was 55 (interquartile range: 44.0, 65.0) years. Detailed baseline characteristics, including baseline psychiatric conditions, are presented in Table 1.
Table 1.
Baseline Characteristics of Study Subjects
| Characteristics | Full Cohort (N = 246) | Baseline VAT < Sample Median (N = 123) | Baseline VAT ≥ Sample Median (N = 123) | P Value (Above vs Below Median Baseline VAT) |
|---|---|---|---|---|
| Age, median (IQR), y | 55.0 (44.0–65.0) | 52 (39–61) | 60 (50–67) | <0.001 |
| Male, N (%) | 100 (40.7) | 33 (26.8) | 67 (54.5) | <0.001 |
| White, N (%) | 222 (90.2) | 109 (88.6) | 113 (91.9) | 0.37 |
| Current smoker, N (%) | 21 (8.5) | 9 (7.3) | 12 (9.8) | 0.29 |
| Hypertension, N (%) | 88 (35.8) | 28 (22.8) | 60 (48.8) | <0.001 |
| Diabetes mellitus, N (%) | 21 (8.5) | 5 (4.1) | 16 (13) | 0.01 |
| Hyperlipidemia, N (%) | 72 (29.3) | 20 (16.3) | 52 (42.3) | <0.001 |
| Total cholesterol, mean (SD), mg/dL | 189.5 (49.4) | 187.1 (48.6) | 191.8 (50.4) | 0.66 |
| LDL, mean (SD), mg/dL | 107.5 (40.2) | 104.7 (40.3) | 110 (40.3) | 0.50 |
| Statin therapy, N (%) | 49 (19.9) | 11 (8.9) | 38 (30.9) | <0.001 |
| FRS, mean (SD) | 6 (6.4) | 3.6 (4.6) | 8.2 (7.0) | <0.001 |
| BMI, median (IQR), kg/m2 | 26.5 (23.4–31.1) | 23.8 (21.4–26.3) | 30 (26.5–33.0) | <0.001 |
| History of cancer, N (%) | 217 (88.2) | 112 (91.1) | 105 (85.4) | 0.13 |
| Prior chemotherapy, N (%) | 200 (81.3) | 103 (83.7) | 97 (78.9) | 0.22 |
| Depression, N (%)a | 12 (4.9) | 6 (4.9) | 6 (4.9) | 1.00 |
| Anxiety, N (%)a | 14 (5.7) | 4 (3.3) | 10 (8.1) | 0.001 |
| Other psychiatric disorders, N (%)a | 2 (0.8) | 1 (0.8) | 1 (0.8) | 1.00 |
Values are mean (SD), median (P25–P75), or N (%). Baseline VAT measurements were available in 246 subjects. Bold P values are <0.05.
Abbreviations: FRS, Framingham Risk Score; IQR, interquartile range; LDL, low-density lipoprotein.
Data on baseline psychiatric disease available in 242 subjects. Other psychiatric diseases include posttraumatic stress disorder (N = 1) and obsessive-compulsive disorder (N = 1).
Amygdalar activity was associated with adiposity at baseline
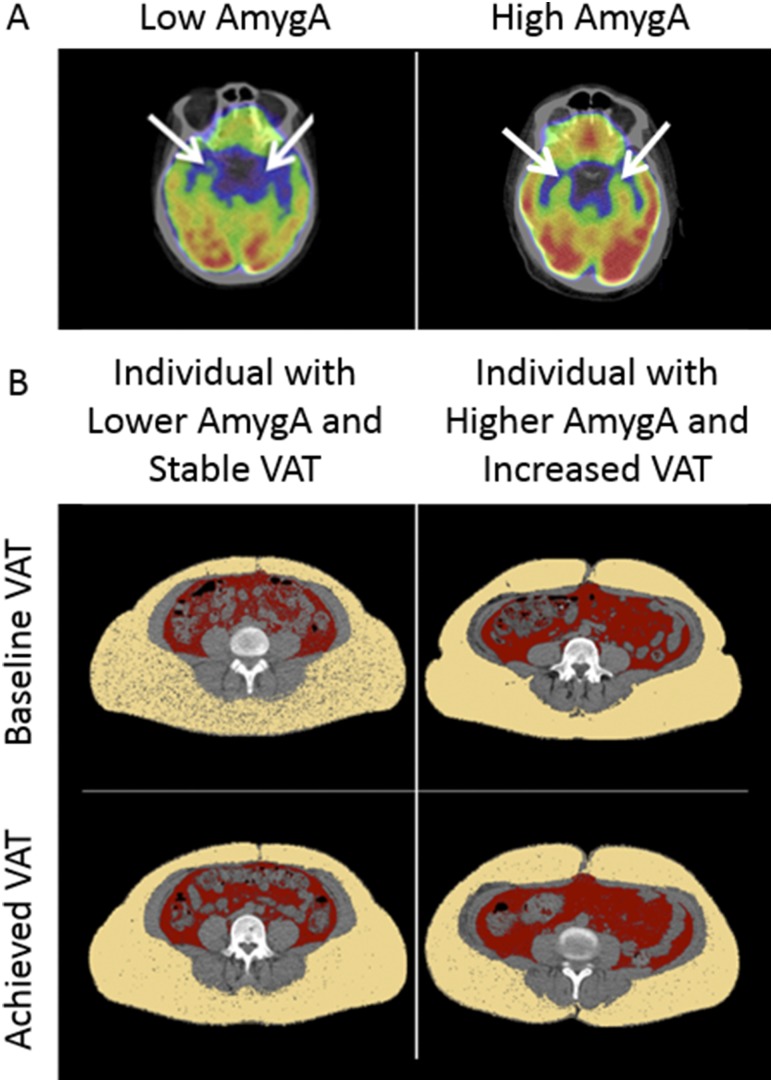
As shown in Table 2, baseline AmygA was associated with baseline BMI [standardized β (95% CI) = 0.15 (0.03, 0.26); P = 0.01]; VAT [0.19 (0.07, 0.30); P = 0.002]; and VAT/SAT ratio [0.20 (0.07, 0.30); P = 0.002]. These associations remained significant after correction for age and sex. In addition, we observed that the associations were unlikely to be modified by habituation (36, 37). Habituation can be broadly conceptualized as an attenuated neural response to stress or fearful stimuli resulting from prior exposure to the imaging test. We observed that the associations between AmygA and baseline adiposity remained significant after adjustment for a history of prior PET/CT imaging: BMI [0.14 (0.02, 0.27); P = 0.03]; VAT [0.13 (0.02, 0.24); P = 0.03]; and VAT/SAT ratio [0.12 (0.02, 0.21); P = 0.02]. Notably, AmygA did not correlate with baseline SAT (P = 0.54), total fat (P = 0.07), or lean body tissue volume (P = 0.33). Images comparing an individual with low AmygA with an individual with high AmygA are shown in Fig. 2A.
Table 2.
Associations Between Baseline Amygdalar Activity and Baseline Measures of Adiposity
| Predictor | Dependent Variable (End Point) | Covariates | Standardized β (95% CI) | P Value |
|---|---|---|---|---|
| Amygdalar activity (continuous) | Baseline BMI, kg/m2 | None | 0.15 (0.03, 0.26) | 0.01 |
| Age and sex | 0.15 (0.04, 0.24) | 0.01 | ||
| Baseline VAT, cm3 | None | 0.19 (0.07, 0.30) | 0.002 | |
| Age and sex | 0.13 (0.02, 0.24) | 0.02 | ||
| Baseline SAT, cm3 | None | 0.04 (−0.08, 0.16) | 0.54 | |
| Age and sex | 0.04 (−0.09, 0.16) | 0.58 | ||
| Baseline VAT/SAT | None | 0.20 (0.07, 0.30) | 0.002 | |
| Age and sex | 0.13 (0.02, 0.22) | 0.02 | ||
| Baseline total fat, cm3 | None | 0.11 (−0.01, 0.23) | 0.07 | |
| Age and sex | 0.09 (−0.04, 0.21) | 0.19 | ||
| Baseline lean tissue volume, cm3 | None | −0.07 (−0.21, 0.07) | 0.33 | |
| Age and sex | 0.02 (−0.06, 0.11) | 0.59 |
Baseline VAT and SAT measurements were available in 246 subjects. Bold P values are <0.05.
Figure 2.
(A) Comparison of an individual with low amygdalar activity with an individual with high amygdalar activity. The arrows identify bilateral amygdalae in each subject. (B) Comparison of baseline and achieved visceral adipose tissue in an individual with lower baseline amygdalar activity with that of an individual with higher baseline amygdalar activity.
Amygdalar activity was associated with subsequent gain in adiposity
In the subset of 68 subjects who underwent follow-up 18F-FDG PET/CT imaging (after ∼1 year), baseline AmygA was associated with achieved VAT [standardized β (95% CI) = 0.19 (0.03, 0.34); P = 0.02] and weight [0.24 (0.12, 0.37); P < 0.001] after adjustment for age, sex, and baseline adiposity (Table 3). Similarly, there was a significant relationship between AmygA and subsequent change in adiposity, including VAT and total fat volume, in univariable models and in models adjusted for age, sex, and baseline adiposity (Table 4). Adjustment for a history of prior imaging did not change the significance of the associations. Images that compare a subject with lower AmygA and stable VAT with an individual with higher AmygA and increased VAT are shown in Fig. 2B.
Table 3.
Associations Between Baseline Amygdalar Activity and Achieved Adiposity Over 1 Year
| Amygdalar Activity (Continuous) |
||
|---|---|---|
| Standardized β (95% CI)a | P Value | |
| Achieved weight, kg | 0.24 (0.12, 0.37) | <0.001 |
| Achieved VAT, cm3 | 0.19 (0.03, 0.34) | 0.02 |
| Achieved SAT, cm3 | 0.09 (−0.09, 0.27) | 0.31 |
| Achieved VAT/SAT | −0.05 (−0.26, 0.15) | 0.61 |
| Achieved total fat, cm3 | 0.13 (−0.05, 0.31) | 0.14 |
| Lean tissue volume, cm3 | −0.02 (−0.12, 0.08) | 0.66 |
Serial VAT and SAT measurements were available in 68 subjects. Bold P values are <0.05.
Corrected for age, sex, and baseline adiposity (achieved weight adjusted for baseline BMI).
Table 4.
Associations Between Baseline Amygdalar Activity and Change in Adiposity Measurements Over 1 Year
| Independent Variable |
Dependent Variable (End Point) |
Dependent Variable (End Point) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Δ Weight (kg) |
Δ VAT (cm3) |
Δ SAT (cm3) |
Δ VAT/SAT |
Δ Total Fat (cm3) |
Δ Lean Tissue Volume (cm3) |
||||||||
| Predictor | Covariate | Standardizedβ (95% CI) | P Value | Standardized β (95% CI) | P Value | Standardized β (95% CI) | P Value | Standardized β (95% CI) | P Value | Standardized β (95% CI) | P Value | Standardized β (95% CI) | P Value |
| Amygdalar activity (continuous) | Univariate | −0.09 | 0.19 | 0.65 | <0.001 | 0.38 | 0.05 | 0.15 | 0.43 | 0.50 | 0.009 | −0.16 | 0.41 |
| (−0.22, 0.04) | (0.30, 1.00) | (0.01, 0.75) | (−0.23, 0.52) | (0.13, 0.86) | (−0.53, 0.22) | ||||||||
| Age and sex | −0.05 | 0.50 | 0.70 | <0.001 | 0.37 | 0.06 | 0.20 | 0.27 | 0.50 | 0.01 | −0.18 | 0.35 | |
| (−0.18, 0.09) | (0.36, 1.05) | (−0.02, 0.75) | (−0.16, 0.55) | (0.13, 0.87) | (−0.56, 0.20) | ||||||||
| Baseline BMI | −0.08 | 0.28 | 0.67 | <0.001 | 0.40 | 0.04 | 0.14 | 0.45 | 0.52 | 0.006 | −0.13 | 0.47 | |
| (−0.21, 0.06) | (0.31, 1.02) | (0.03, 0.77) | (−0.24, 0.52) | (0.16, 0.88) | (−0.49, 0.23) | ||||||||
| Baseline VAT | −0.06 | 0.41 | 0.65 | 0.001 | 0.36 | 0.06 | 0.16 | 0.41 | 0.48 | 0.01 | −0.20 | 0.29 | |
| (−0.19, 0.08) | (0.29, 1.00) | (−0.02, 0.74) | (−0.22, 0.54) | (0.11, 0.85) | (−0.56, 0.17) | ||||||||
| Baseline SAT | −0.08 | 0.25 | 0.67 | <0.001 | 0.39 | 0.03 | 0.15 | 0.43 | 0.51 | 0.004 | −0.17 | 0.37 | |
| (−0.21, 0.06) | (0.33, 1.01) | (0.04, 0.75) | (−0.23, 0.53) | (0.17, 0.86) | (−0.55, 0.21) | ||||||||
| Baseline VAT/SAT | −0.07 | 0.33 | 0.67 | <0.001 | 0.39 | 0.05 | 0.14 | 0.44 | 0.51 | 0.008 | −0.19 | 0.32 | |
| (−0.21, 0.07) | (0.31, 1.02) | (0.01, 0.77) | (−0.22, 0.49) | (0.13, 0.88) | (−0.57, 0.19) | ||||||||
| Baseline total fat | −0.07 | 0.29 | 0.65 | <0.001 | 0.37 | 0.05 | 0.15 | 0.42 | 0.49 | 0.008 | −0.18 | 0.35 | |
| (−0.21, 0.06) | (0.31, 1.00) | (0.01, 0.74) | (−0.23, 0.53) | (0.13, 0.84) | (−0.55, 0.19) | ||||||||
Serial VAT and SAT measurements were available in 68 subjects. Bold P values are <0.05.
The association between amygdalar activity and VAT was mediated by increased bone marrow activity
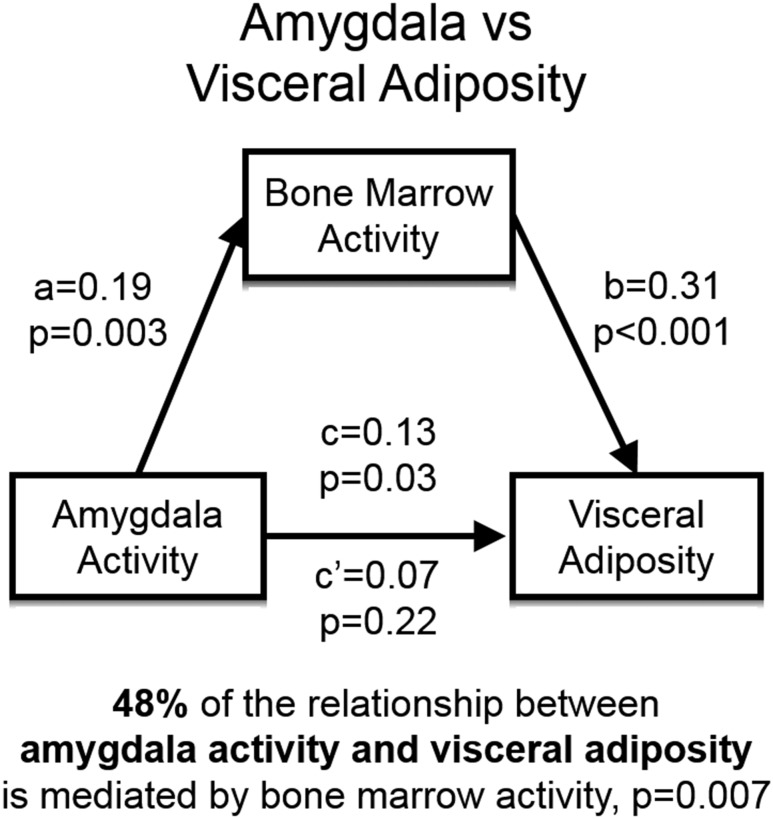
In addition to its associations with VAT, AmygA was also significantly associated with hematopoietic tissue activity, measured as bone marrow 18F-FDG uptake [standardized β (95% CI) = 0.15 (0.04, 0.27); P = 0.01]. In addition, bone marrow activity was closely associated with VAT [0.34 (0.21, 0.44); P < 0.001]. Therefore, we performed mediation (path) analyses to test the hypothesis that the relationship between baseline AmygA and baseline VAT was significantly mediated by upregulated bone marrow activity. The results of the mediation analysis (Fig. 3) indicated that bone marrow activity was a significant mediator of the relationship between AmygA and VAT [0.06 (0.01, 0.13); P = 0.007], accounting for 48% of the total effect. This suggests that bone marrow activity plays a critical role in mediating the association between AmygA and VAT.
Figure 3.
Mediation model for hypothesized pathway from baseline amygdalar metabolic activity to baseline visceral adiposity. A single mediator analysis demonstrated that bone marrow activity was a significant mediator [standardized β (95% CI) = 0.06 (0.007, 0.13); P = 0.007] of the relationship between baseline AmygA and baseline VAT, accounting for 48% of the total effect. Standardized regression coefficients are shown. Analyses included age and sex as covariates. a, effect of AmygA on bone marrow activity; b, effect of bone marrow activity on VAT; c, total effect of AmygA on VAT; c’, residual direct effect of AmygA on VAT (independent of mediated effects).
Discussion
The current study provides insights into the neurobiological mechanism by which chronic stress associates with adiposity. We observed in humans that metabolic activity of a brain region involved in the conditioned emotional and physiological responses to stress (i.e., AmygA) independently links to baseline as well as achieved VAT. Our observations further suggest that AmygA links to adiposity via a path that involves upregulation of hematopoiesis (presumably leukopoiesis), as reflected in bone marrow activity. Together, the study findings illuminate a potential biological pathway involving inflammation that links amygdalar activity to adiposity in humans.
Mechanistic insights
VATs are metabolically active and rich in immune cells. In obese individuals, functionally impaired hypertrophic adipocytes are a source of proinflammatory cytokines and chemokines such as TNF-α, IL-6, and monocyte chemoattractant protein-1 (38–40). In particular, MCP-1 promotes the migration of bone marrow‒derived monocytes and macrophages (41) and leads to differential activation of adipose tissue macrophages and modulation of local inflammation in VAT (42). In addition, macrophage infiltration has been strongly associated with further VAT accumulation (43). These factors promote a chronic, low-grade, systemic inflammatory state affecting multiple tissues, which is associated with increased prevalence of chronic diseases such as atherosclerosis (38). Animal studies have shown that unpredictable chronic stress promotes increased local and systemic inflammation and stimulates alterations in adipose tissue with increases in VAT (11). The current study provides support for a homologous pathogenic mechanism in humans.
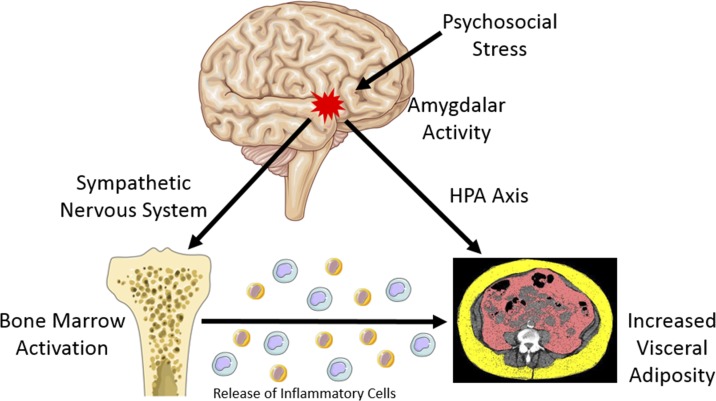
The current study underscores the role of the amygdala as a key neural participant in the mechanism by which the emotional and physiological response to chronic stress induces VAT. Amygdalar activation prompts activation of the hypothalamic-pituitary-adrenal (HPA) axis (44) and sympathetic nervous system (SNS) (45), which were not directly studied in this cohort. Activation of the HPA axis and increased sympathetic tone facilitate the release of fatty acids from fat stores to increase the systemic energy supply in response to stress (46). Monocytes within fat release cytokines that subsequently modify the glucose transporter ratio (glut4/glut1) to allow glucose entry into adipocytes in the context of SNS suppression of insulin (22). Simultaneously, increased glucocorticoids and other stress hormones may lead to upregulation of neuropeptide Y and induce differentiation of progenitors into adipocytes, thereby increasing the absolute number of adipocytes (47).
The current study suggests an important physiological connection between the amygdala and hematopoietic tissues in promoting visceral adiposity. The amygdala’s projections to the brainstem play a key role in the response of the SNS to stress (45). In murine models, through stimulation of sympathetic nerves terminating in the bone marrow, stress leads to increased hematopoietic stem and progenitor cell proliferation and accelerates innate immune cell output and cytokine production (16–20). In the setting of obesity, these bone marrow‒derived monocytes have been shown to migrate to and accumulate in VAT (41), leading to alterations in the balance of proinflammatory and anti-inflammatory macrophages and increases in local inflammation within VAT (42). This study further supports an important role for hematopoietic tissues in the mechanism linking neurobiological activity to metabolic disease by suggesting that in humans increased bone marrow activity accounts for nearly half of the association between baseline AmygA and baseline VAT. Accordingly, the findings of the current study provide key extensions to the existing literature by illuminating a potential mechanism that translates a neurobiological response to chronic stress into excess VAT (Fig. 4).
Figure 4.
Proposed mechanism linking amygdalar activity to increased visceral adipose tissue.
Limitations
The individuals in the study were selected from a clinical database of patients who had undergone 18F-FDG PET/CT for clinical indications, mainly cancer screening and surveillance, thus potentially limiting the generalizability of the findings. Subjects were not prospectively recruited, and the results are subject to the inherent limitations of a retrospective single-center design. We did not have the opportunity to account for health behaviors such as diet and physical activity, which may confound the relationship between chronic stress and adiposity. There was also no opportunity to compare the imaging findings with measurements of cytokines, neurohormones, and inflammatory biomarkers, given that these factors were not routinely measured in this clinical population. Standard questionnaires to assess perceived stress were not used, so the relationship between perceived stress and adiposity was not directly evaluated. Because many subjects had undergone 18F-FDG PET/CT imaging before baseline study imaging, unmeasured amygdalar habituation, a decrement in amygdalar response with repeated exposure to a stimulus (e.g., facial expressions) (36, 37), may have occurred in some individuals. However, because resting amygdalar activity on 18F-FDG PET/CT imaging is stable in individuals with repeat scanning (29, 30), resting rather than provocative imaging was performed, and the results are robust to adjustment for 18F-FDG PET/CT imaging before study entry, providing reassurance that habituation did not contribute substantially to these findings. Finally, because of the limitations of resolution and limited standard brain coverage with whole-body 18F-FDG PET/CT imaging, we were unable to evaluate the activity of several other important stress-responsive neural centers (e.g., the hippocampus, prefrontal cortex). Nevertheless, in this study, AmygA was found to be strongly associated with baseline and achieved VAT, and the previously cited limitations are substantially counterbalanced by the novelty of the findings.
Future perspectives
These findings present opportunities for several important future studies. Pharmacologic modification of the discovered pathologic mechanism may be possible and could include targeting brain centers involved in the perception of and response to stress, bone marrow activation, and/or resultant monocytosis. Nonpharmacologic interventions could be equally attractive. It has previously been demonstrated that stress-reduction may have a role in modifying the neurobiological milieu (48) as well as amygdalar structure and function (49, 50), suggesting a possible role for stress-reduction techniques in interrupting this pathogenic pathway. In addition, the roles of health behaviors, the SNS, and the HPA axis in mediating the link between AmygA and adiposity should be directly studied in future investigations. Similarly, a comprehensive evaluation of inflammatory activity (e.g., measurement of cytokines and inflammatory biomarkers) should be performed to further assess the role of hematopoietic tissue in this pathway. Complete neuroimaging with hybrid 18F-FDG PET and MRI should also be considered to allow reproducible assessment of all involved neural centers, and the possible effect of habituation on resting AmygA should be assessed. Finally, these findings suggest a potential role for screening patients who are overweight for chronic stress and stress conditions to optimize and individualize therapeutic interventions.
Conclusion
The current study illustrates a relationship in humans between AmygA and adiposity, notably VAT. Moreover, we demonstrated that this link between AmygA and VAT is significantly mediated by increased bone marrow activity (indicative of greater leukopoiesis), thereby implicating inflammation in this mechanism. Future studies may lead to novel insights on how to treat obesity and prevent downstream disease by modulating this pathway.
Acknowledgments
The Harvard Catalyst Statistical Consulting Service and Sarah Mercaldo assisted with statistical methods.
Financial Support: This work is supported in part by the following NIH grants: T32HL076136 (to M.T.O.), P01HL131478 (to Z.A.F.), P30DK040561 (to S.K.G.), and R01HL122177 (to A.T.).
Disclosure Summary: A.T. received institutional grants from Genentech and Actelion and personal fees from Actelion during the conduct of this study for research outside the submitted work. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- 18F
fluorodeoxyglucose positron emission tomography/computed tomography
- AmygA
activity of the amygdala
- BMI
body mass index
- CVD
cardiovascular disease
- HPA
hypothalamic-pituitary-adrenal
- PET
positron emission tomography
- SAT
subcutaneous adipose tissue
- SNS
sympathetic nervous system
- SUV
standardized uptake value
- VAT
visceral adipose tissue
References
- 1. Obesity: preventing and managing the global epidemic: report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii, 1–253. [PubMed] [Google Scholar]
- 2. Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA. 2010;303(3):242–249. [DOI] [PubMed] [Google Scholar]
- 3. Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67(5):968–977. [DOI] [PubMed] [Google Scholar]
- 4. Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62(10):921–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P Jr, Razak F, Sharma AM, Anand SS; INTERHEART Study Investigators . Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366(9497):1640–1649. [DOI] [PubMed] [Google Scholar]
- 7. Björntorp P. Abdominal fat distribution and disease: an overview of epidemiological data. Ann Med. 1992;24(1):15–18. [DOI] [PubMed] [Google Scholar]
- 8. Canoy D, Boekholdt SM, Wareham N, Luben R, Welch A, Bingham S, Buchan I, Day N, Khaw KT. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population-based prospective study. Circulation. 2007;116(25):2933–2943. [DOI] [PubMed] [Google Scholar]
- 9. Barrington WE, Ceballos RM, Bishop SK, McGregor BA, Beresford SA. Perceived stress, behavior, and body mass index among adults participating in a worksite obesity prevention program, Seattle, 2005-2007. Prev Chronic Dis. 2012;9:120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wardle J, Chida Y, Gibson EL, Whitaker KL, Steptoe A. Stress and adiposity: a meta-analysis of longitudinal studies. Obesity (Silver Spring). 2011;19(4):771–778. [DOI] [PubMed] [Google Scholar]
- 11. Karagiannides I, Golovatscka V, Bakirtzi K, Sideri A, Salas M, Stavrakis D, Polytarchou C, Iliopoulos D, Pothoulakis C, Bradesi S. Chronic unpredictable stress regulates visceral adipocyte-mediated glucose metabolism and inflammatory circuits in male rats. Physiol Rep. 2014;2(5):e00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bartolomucci A, Cabassi A, Govoni P, Ceresini G, Cero C, Berra D, Dadomo H, Franceschini P, Dell’Omo G, Parmigiani S, Palanza P. Metabolic consequences and vulnerability to diet-induced obesity in male mice under chronic social stress. PLoS One. 2009;4(1):e4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stewart-Knox B, E Duffy M, Bunting B, Parr H, Vas de Almeida MD, Gibney M. Associations between obesity (BMI and waist circumference) and socio-demographic factors, physical activity, dietary habits, life events, resilience, mood, perceived stress and hopelessness in healthy older Europeans. BMC Public Health. 2012;12(1):424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: prospective study. BMJ. 2006;332(7540):521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aschbacher K, Kornfeld S, Picard M, Puterman E, Havel PJ, Stanhope K, Lustig RH, Epel E. Chronic stress increases vulnerability to diet-related abdominal fat, oxidative stress, and metabolic risk. Psychoneuroendocrinology. 2014;46:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bernberg E, Ulleryd MA, Johansson ME, Bergström GM. Social disruption stress increases IL-6 levels and accelerates atherosclerosis in ApoE-/- mice. Atherosclerosis. 2012;221(2):359–365. [DOI] [PubMed] [Google Scholar]
- 17. Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487(7407):325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Emami H, Singh P, MacNabb M, Vucic E, Lavender Z, Rudd JH, Fayad ZA, Lehrer-Graiwer J, Korsgren M, Figueroa AL, Fredrickson J, Rubin B, Hoffmann U, Truong QA, Min JK, Baruch A, Nasir K, Nahrendorf M, Tawakol A. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC Cardiovasc Imaging. 2015;8(2):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, Lin CP, Vinegoni C, Libby P, Swirski FK, Weissleder R, Nahrendorf M. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20(7):754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang T, Chen Y, Liu H, Zhou Z, Zhai Y, Yang J. Chronic unpredictable stress accelerates atherosclerosis through promoting inflammation in apolipoprotein E knockout mice. Thromb Res. 2010;126(5):386–392. [DOI] [PubMed] [Google Scholar]
- 21. Speaker KJ, Fleshner M. Interleukin-1 beta: a potential link between stress and the development of visceral obesity. BMC Physiol. 2012;12(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peters A, McEwen BS. Stress habituation, body shape and cardiovascular mortality. Neurosci Biobehav Rev. 2015;56:139–150. [DOI] [PubMed] [Google Scholar]
- 23. Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med. 2005;35(6):791–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62(3):273–281. [DOI] [PubMed] [Google Scholar]
- 25. Tawakol A, Ishai A, Takx RAP, Figueroa AL, Ali A, Kaiser Y, Truong QA, Solomon CJE, Calcagno C, Mani V, Tang CY, Mulder WJM, Murrough JW, Hoffmann U, Nahrendorf M, Shin LM, Fayad ZA, Pitman RK. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet. 2017;389(10071):834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Osborne MT, Ishai A, Hammad B, Tung B, Wang Y, Baruch A, Fayad ZA, Giles JT, Lo J, Shin LM, Grinspoon SK, Koenen KC, Pitman RK, Tawakol A. Amygdalar activity predicts future incident diabetes independently of adiposity. Psychoneuroendocrinology. 2018;100:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gray TS, Bingaman EW. The amygdala: corticotropin-releasing factor, steroids, and stress. Crit Rev Neurobiol. 1996;10(2):155–168. [DOI] [PubMed] [Google Scholar]
- 28. Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24(24):5506–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hammad B. T Osborne M, Ishai A, Wang Y, Tung B, Baruch A, Klimas M, M Shin L, Fayad Z, T Glies J, Pitman R, Tawakol A. Increased stress-related neural tissue activity potentiates inflammation and impedes the anti-inflammatory impact of statins Circulation. 2017;136(Suppl 1):A18768 Abstract 18768. [Google Scholar]
- 30. Schaefer SM, Abercrombie HC, Lindgren KA, Larson CL, Ward RT, Oakes TR, Holden JE, Perlman SB, Turski PA, Davidson RJ. Six-month test-retest reliability of MRI-defined PET measures of regional cerebral glucose metabolic rate in selected subcortical structures. Hum Brain Mapp. 2000;10(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu Y, Du R, Zhu Y, Shen Y, Zhang K, Chen Y, Song F, Wu S, Zhang H, Tian M. PET mapping of neurofunctional changes in a posttraumatic stress disorder model. J Nucl Med. 2016;57(9):1474–1477. [DOI] [PubMed] [Google Scholar]
- 32. Oler JA, Fox AS, Shelton SE, Rogers J, Dyer TD, Davidson RJ, Shelledy W, Oakes TR, Blangero J, Kalin NH. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 2010;466(7308):864–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Whalen PJ, Shin LM, Somerville LH, McLean AA, Kim H. Functional neuroimaging studies of the amygdala in depression. Semin Clin Neuropsychiatry. 2002;7(4):234–242. [DOI] [PubMed] [Google Scholar]
- 34. Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O’Donnell CJ, Hoffmann U. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes. 2007;31(3):500–506. [DOI] [PubMed] [Google Scholar]
- 35. Britz-Cunningham SH, Millstine JW, Gerbaudo VH. Improved discrimination of benign and malignant lesions on FDG PET/CT, using comparative activity ratios to brain, basal ganglia, or cerebellum. Clin Nucl Med. 2008;33(10):681–687. [DOI] [PubMed] [Google Scholar]
- 36. Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17(5):875–887. [DOI] [PubMed] [Google Scholar]
- 37. Fischer H, Wright CI, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Brain habituation during repeated exposure to fearful and neutral faces: a functional MRI study. Brain Res Bull. 2003;59(5):387–392. [DOI] [PubMed] [Google Scholar]
- 38. Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288(5):H2031–H2041. [DOI] [PubMed] [Google Scholar]
- 39. Ghigliotti G, Barisione C, Garibaldi S, Fabbi P, Brunelli C, Spallarossa P, Altieri P, Rosa G, Spinella G, Palombo D, Arsenescu R, Arsenescu V. Adipose tissue immune response: novel triggers and consequences for chronic inflammatory conditions. Inflammation. 2014;37(4):1337–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pietiläinen KH, Róg T, Seppänen-Laakso T, Virtue S, Gopalacharyulu P, Tang J, Rodriguez-Cuenca S, Maciejewski A, Naukkarinen J, Ruskeepää AL, Niemelä PS, Yetukuri L, Tan CY, Velagapudi V, Castillo S, Nygren H, Hyötyläinen T, Rissanen A, Kaprio J, Yki-Järvinen H, Vattulainen I, Vidal-Puig A, Orešič M. Association of lipidome remodeling in the adipocyte membrane with acquired obesity in humans. PLoS Biol. 2011;9(6):e1000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chinetti-Gbaguidi G, Staels B. Macrophage polarization in metabolic disorders: functions and regulation. Curr Opin Lipidol. 2011;22(5):365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Michaud A, Drolet R, Noël S, Paris G, Tchernof A. Visceral fat accumulation is an indicator of adipose tissue macrophage infiltration in women. Metabolism. 2012;61(5):689–698. [DOI] [PubMed] [Google Scholar]
- 44. Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002;71(3):431–447. [DOI] [PubMed] [Google Scholar]
- 45. LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8(7):2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kubera B, Hubold C, Zug S, Wischnath H, Wilhelm I, Hallschmid M, Entringer S, Langemann D, Peters A. The brain’s supply and demand in obesity. Front Neuroenergetics. 2012;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R, Herzog H, Zukowska Z. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med. 2007;13(7):803–811. [DOI] [PubMed] [Google Scholar]
- 48. Kiecolt-Glaser JK, Christian LM, Andridge R, Hwang BS, Malarkey WB, Belury MA, Emery CF, Glaser R. Adiponectin, leptin, and yoga practice. Physiol Behav. 2012;107(5):809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hölzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, Morgan L, Pitman RK, Lazar SW. Stress reduction correlates with structural changes in the amygdala. Soc Cogn Affect Neurosci. 2010;5(1):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Goldin PR, Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion. 2010;10(1):83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]