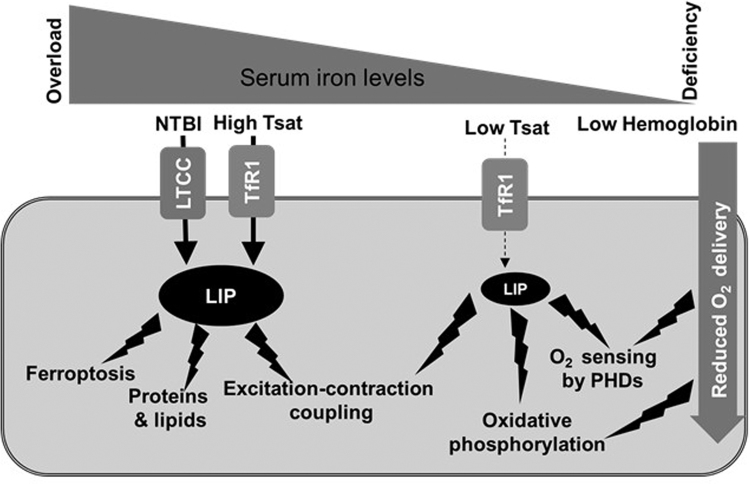
Fig. 1.
Effects of systemic iron imbalance on the cardiomyocyte. In conditions of iron overload, transferrin saturation (Tsat) is elevated, and non-transferrin bound iron NTBI increases in the circulation. NTBI (in the form of Fe 2+) is taken up into cardiomyocytes by L-type calcium channels (LTCC), which are not regulated in response to intracellular iron overload. Higher levels of iron uptake increase the size of the labile iron pool (LIP) to levels that generate excessive reactive oxygen species (ROS). These damage proteins and lipids and interfere with excitation-contraction coupling, leading to diastolic dysfunction. In addition, increased LIP could promote ferroptosis following injury (e.g. ischemia reperfusion injury, cardiac haemorrhage). In conditions of iron deficiency, the iron available for uptake by cardiomyocytes is reduced because of lower Tsat. This results in a reduction in the size of LIP, limiting the availability of iron for the synthesis of enzymes involved in oxidative phosphorylation. Other effects may also include impaired redox signalling and oxygen sensing by HIF prolyl hydroxylases, which utilise iron as a co-factor. When iron deficiency is accompanied by anaemia, lower haemoglobin levels can result in reduced oxygen delivery to the cardiomyocyte. This in turn also affects oxygen sensing and oxidative phosphorylation.