Figure 7.
BRD3 Impacts rRNA Production and Cell Proliferation
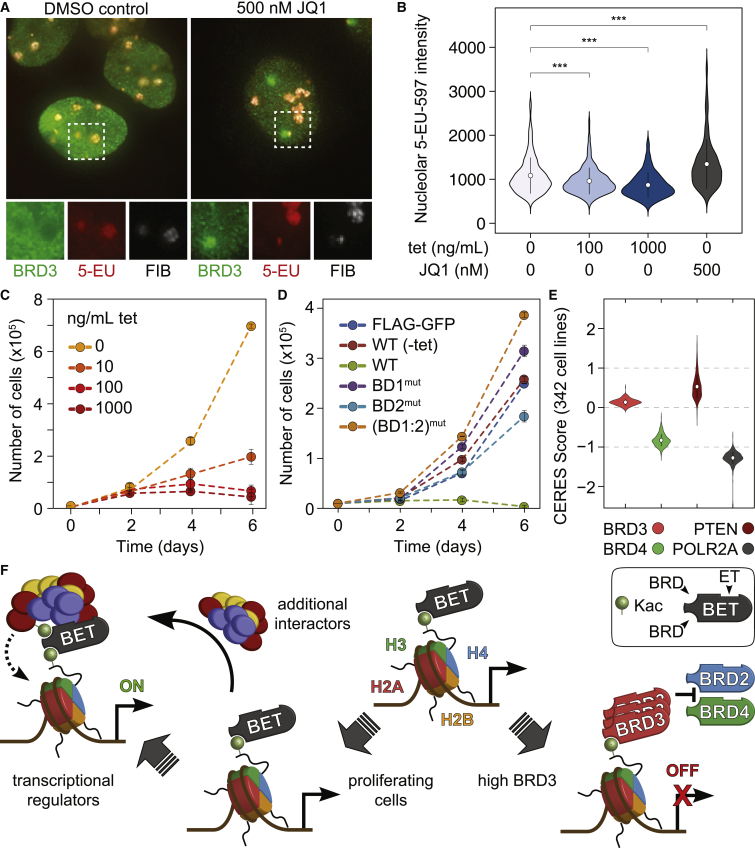
(A) U-2 OS cells were treated with JQ1 or DMSO for 1 hr and then fed 5-EU for 1 hr prior to staining for BRD3, fibrillarin (FIB), and click chemistry to 5-EU-labeled RNA.
(B) Quantitative immunofluorescence of U-2 OS cells treated with various concentrations of tetracycline (to titrate BRD3 levels) or JQ1. 5-EU signal overlapping with fibrillarin signal (i.e., nucleolar RNA) was quantified for >400 cells for each experimental condition. ∗∗∗p value <0.001, by two-tailed Student’s t test.
(C) Cell proliferation assay for U-2 OS treated with tetracycline over 6 days (n = 3).
(D) Cell proliferation assay for U-2 OS cells induced with 1 μg/mL tetracycline over 6 days (n = 3).
(E) Violin plots of CERES scores showing gene depletion effects from CRISPR-Cas9 loss-of-function screens in 342 cancer cell lines (Meyers et al., 2017).
(F) Model of BET protein recruitment functions and the impact of BRD3 overexpression. In proliferating cells, BETs bind to acetylated proteins, including histones through their tandem BRDs, and recruit to chromatin additional transcriptional regulators through other modular domains. High levels of BRD3 antagonize this by decreasing the levels of other BETs, and competing for binding to common loci, in addition to reducing rRNA levels, with a net result of decreasing proliferation.