Figure 6. A model of the tripartite synapse.
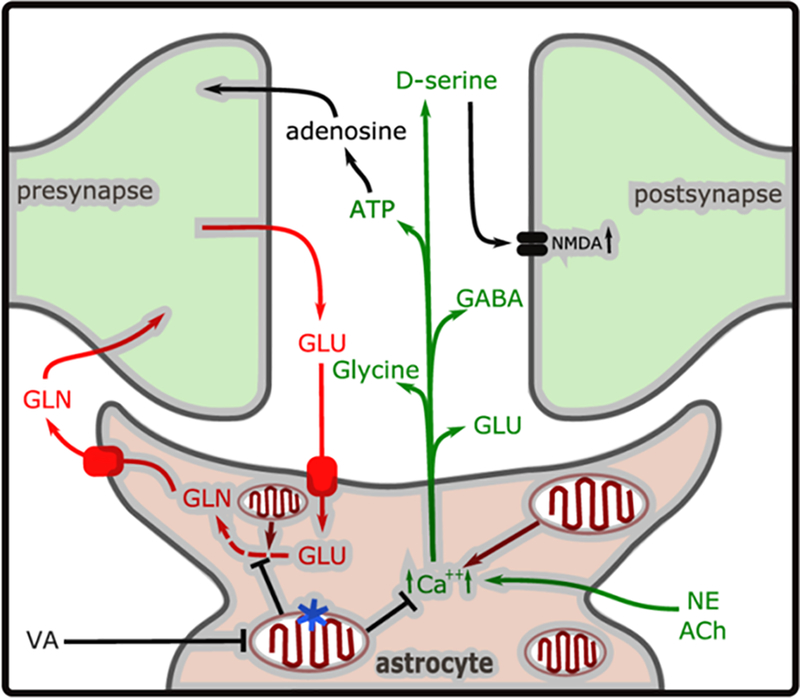
Glutamate (GLU) released during synaptic transmission is taken up by GLT-1 or GLAST transporters, converted to glutamine (GLN) and released by SN1/SN2 (pathway indicated in red). In addition, NE (norepinephrine) or ACh (acetylcholine) stimulation during emergence causes increased intracellular Ca2+ concentration ([Ca2+]i), causing release of the gliotransmitters ATP, GLU, and D-serine, glycine and GABA (in green). The Ca2+ homeostasis or glutamate recycling may be dysregulated in the astrocyte-specific mitochondrial mutant (indicated by blue star). The volatile anesthetics (ISO/HAL) inhibit mitochondrial complex I function at a lower concentration in Ndufs4(KO) mitochondria than in control mitochondria. In the case of the astrocyte specific Ndufs4(KO) (mutation indicated by the blue star in one mitochondrion), this leads to mitochondrial inhibition persisting in the astrocyte, compared to the neurons, during reduction of anesthetic concentration. We propose that this leads to neuronal inhibition from decreased excitatory gliotransmitter release or defective glutamate recycling, resulting in delayed emergence.
