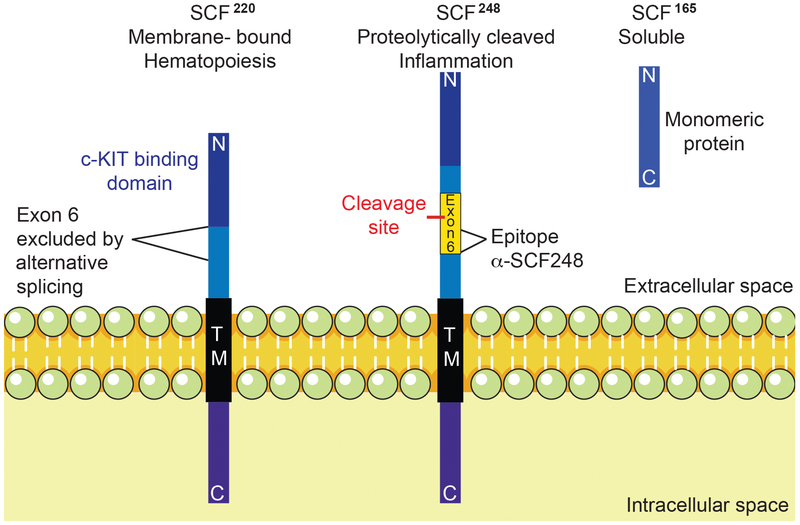
Figure 1. SCF isoforms and α-SCF248.
Endogenous SCF is found in 2 primary forms, SCF220 and SCF248, both are initially found as transmembrane proteins, which require dimerization to activate their receptor c-KIT. SCF220 isoform is critical for hematopoiesis, while SCF248 is related to the inflammatory process. Only the SCF248 form has an enzyme cleavable domain, which allows it to be released from the surface of the cell to generate soluble SCF165 (found as a monomeric isoform that is unable to activate c-KIT in vivo16). These two splice variants differ by the presence or absence of exon 6, which encodes the enzyme cleavable domain found in the isoform SCF248. Monoclonal α-SCF248 antibodies detect an epitope on the membrane side of the cleavage in exon 6. Thus, the monoclonal only recognizes SCF248 as an intact membrane associate protein and not the soluble SCF165 form.