Figure 1. Signalling in hypoxic pulmonary vasoconstriction.
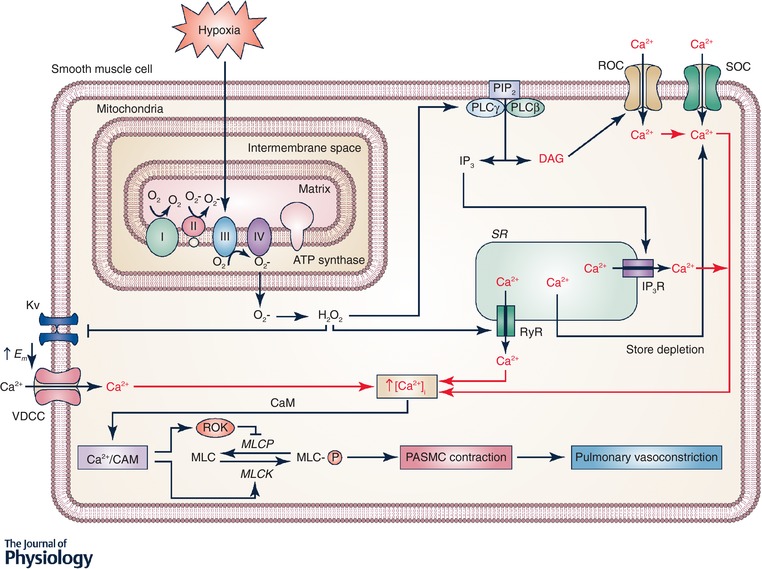
Hypoxia increases production at mitochondrial complex III of reactive oxygen species (ROS), which are released into the mitochondrial intermembrane space. ROS signals (superoxide and H2O2) can move from the intermembrane space to the cytosol where the superoxide is converted to H2O2. H2O2 can then activate many downstream signalling pathways. H2O2 can activate phospholipase C (PLC) leading to production of inositol 1,4,5‐trisphosphate (IP3) and diacylglycerol (DAG) from phosphatidylinositol bisphosphate (PIP2). IP3 activates IP3 receptors resulting in Ca2+ release from the sarcoplasmic reticulum (SR) and DAG activates receptor‐operated Ca2+ channels (ROCC). H2O2 also directly activates ryanodine receptors (RyR) by oxidizing cysteine residues, leading the Ca2+ release from SR. Release of Ca2+ from the SR causes store depletion, which activates Ca2+ entry via activation of calcium release‐activated Ca2+ (CRAC) channels. H2O2 also inhibits KV channels, which increases the membrane potential (E m) and opens voltage‐dependent Ca2+ channels (VDCC). Together, the increased intracellular Ca2+ concentration ([Ca2+]i) causes Ca2+ to bind calmodulin (CaM) and activate myosin light chain kinase (MLCK), which phosphorylates myosin (MLC) and leads to contraction of pulmonary arterial smooth muscle cells (PASMC).
